Abstract
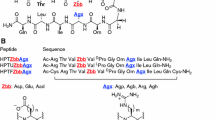
THE three-dimensional structures of proteins reveal that the distribution of amino acids within the major classes of secondary structure is not random but that each amino acid has its own preferred secondary structural arrangements1–5. Propensity scales for residues in α-helices have been generated through the use of various host-guest systems6–9. Here we measure the thermo-dynamic β -sheet propensities of each of the twenty commonly occurring amino acids. A previously studied zinc-finger peptide10 was used as the host system in which amino acids were substituted into a guest site, a solvent-exposed position in an antiparallel β -sheet. As these peptides are unfolded in the absence of bound metal but are folded in their presence, it is assumed that the thermodynamics of metal binding fully reflect peptide-folding energy. A competitive cobalt(II)-binding assay was used to determine these energies with high precision. The relative free energies correlate well with previously derived potential values based on statistical analysis of protein structures. We are therefore able to present a thermodynamic β -sheet propensity scale for all the commonly occurring amino acids in aqueous solution.
Similar content being viewed by others
References
Chou, P. Y. & Fasman, G. D. Biochemistry 13, 211–222 (1973).
Levitt, M. Biochemistry 17, 4277–4285 (1978).
Garnier, J., Osguthorpe, D. J. & Robson, B. J. molec. Biol. 120, 97–120 (1978).
Lifson, S. & Sander, C. Nature 282, 109–111 (1979).
Fasman, G. D. in Prediction of Protein Structure and the Principles of Protein Conformation (ed. Fasman, G. D.) (Plenum, New York, 1989).
Wojcik, J., Altmann, K.-H. & Scheraga, H. A. Biopolymers 30, 121–134 (1990).
Padmanabhan, S., Marqusee, S., Ridgeway, T., Laue, T. M. & Baldwin, R. L. Nature 344, 268–270 (1990).
O'Neil, K. T. & DeGrado, W. F. Science 250, 646–651 (1990).
Lyu, P. C., Liff, M. I., Marky, L. A. & Kallenbach, N. R. Science 250, 669–673 (1990).
Krizek, B. A., Amann, B. T., Kilfoil, V. J., Merkle, D. L. & Berg, J. M. J. Am. chem. Soc. 113, 4518–4523 (1991).
Harper, L. V., Amann, B. T., Kilfoil, V. J. & Berg, J. M. J. Am. chem. Soc. (in the press).
Frankel, A. D., Berg, J. M. & Pabo, C. O. Proc. natn. Acad Sci. U.S.A. 84, 4841–4845 (1987).
Parraga, G. et al. Science 241, 1489–1492 (1988).
Pavletich, N. P. & Pabo, C. O. Science 252, 809–816 (1991).
Krizek, B. A., Merkle, D. L. & Berg, J. M. Inorg. Chem. (in the press).
Creamer, T. P. & Rose, G. D. Proc. natn. Acad. Sci. U.S.A. 89, 5937–5941 (1992).
McGregor, M. J., Islam, S. A. & Sternberg, M. J. E. J. molec. Biol. 198, 295–310 (1987).
Zawadzke, L. E. & Berg, J. M. J. Am. chem. Soc. 114, 4002–4003 (1992).
Richardson, J. S. Nature 268, 495–500 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, C., Berg, J. Thermodynamic β -sheet propensities measured using a zinc-finger host peptide. Nature 362, 267–270 (1993). https://doi.org/10.1038/362267a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/362267a0
- Springer Nature Limited
This article is cited by
-
Uncovering supramolecular chirality codes for the design of tunable biomaterials
Nature Communications (2024)
-
Specific metallo-protein interactions and antimicrobial activity in Histatin-5, an intrinsically disordered salivary peptide
Scientific Reports (2019)
-
Why Proteins are Big: Length Scale Effects on Equilibria and Kinetics
The Protein Journal (2019)
-
Amyloidogenic motifs revealed by n-gram analysis
Scientific Reports (2017)
-
Populations of the Minor α-Conformation in AcGXGNH2 and the α-Helical Nucleation Propensities
Scientific Reports (2016)