Abstract
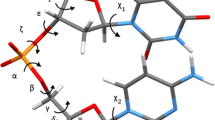
NUCLEIC acids, because of their key biological role, are prime targets for the design of either analogues that may mimic some of their features or of complementary ligands that may selectively bind to and react with them for regulation or reaction. Whereas there has been much work on the latter topic since the elucidation of the double-helical structure of DNA1,2, comparatively little has been done on structural and/or functional models, probably owing to the lack of self-organizing molecular systems. Here we present a class of artificial systems, the nucleohelicates, which are of interest from both points of view because they combine the double-helical structure of the double-stranded metal complexes, the helicates2,3, with the selective interaction features of nucleic-acid bases. These functionalized species allow the study of structural effects on the formation of the double helix and on the binding to other entities, in particular to nucleic acids.
Similar content being viewed by others
References
Watson, J. D. & Crick, F. H. C. Nature 171, 737–738 (1953).
Lehn, J-M. et al. Proc. natn. Acad. Sci. U.S.A. 84, 2565–2569 (1987).
Lehn, J-M. & Rigault, A. Angew. Chem., int. Edn Engl. 27, 1095–1097 (1988).
Alpha, B., Anklam, E., Deschenaux, R., Lehn, J-M. & Pietraskiewicz, M. Helv. chim. Acta 71, 1042–1052 (1988).
Schneider, K. C. & Benner, S. A. J. Am. chem. Soc. 112, 453–455 (1990).
Hoffmann, S., Witkowski, W. & Schubert, H. Z. Chem. 14, 154 (1974).
Takenvoto, K., Kawabuko, F. & Kondo, K. Makromol. Chem. 148, 131–134 (1971).
Browne, D. T., Eisinger, J. & Leonard, N. J. J. Am. chem. Soc. 90, 7302–7323 (1968).
Leonard, N. J. Acc. chem. Res. 12, 423–429 (1979).
Golankiewicz, K. & Celewicz, L. Polish J. Chem. 52, 1035–1038 (1978).
Sasaki, I., Dufour, M-N. & Gaudemer, A. Nouv. J. Chem. 6, 341–344 (1982).
Saito, I., Sugiyama, H., Matsuura, T. & Fukuyama, K. Tetrahedron Lett. 4467–4470 (1985).
Kim, M. & Gokel, G. JCS Chem. Commun. 1686–1688 (1987).
Sessler, J. L., Magdal, D. & Hugdall, J. J. Inclus. Phen. molec. Recogn. 7, 19–26 (1989).
Pauling, L. & Corey, R. B. Nature 171, 346 (1953).
Lehn, J-M. Angew. Chem., int. Edn Engl. 27, 89–112 (1988).
Felsenfeld, G., Danies, D. R. & Rich, A. J. Am. chem. Soc. 79, 2023–2024 (1957).
Moser, H. E. & Dervan, P. E. Science 238, 645–650 (1987).
Francois, J-C., Saison-Behmoaras, T. & Helene, C. Nucleid Acids Res. 16, 11431–11440 (1988).
Rodriguez-Ubis, J-C., Alpha, B., Plancherel, D. & Lehn, J-M. Helv. chim. Acta 67, 2264–2269 (1984).
Banwarth, W. Helv. chim. Acta 71, 1517–1527 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koert, U., Harding, M. & Lehn, JM. DNH deoxyribonucleohelicates: self assembly of oligonucleosidic double-helical metal complexes. Nature 346, 339–342 (1990). https://doi.org/10.1038/346339a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/346339a0
- Springer Nature Limited
This article is cited by
-
Single helically folded aromatic oligoamides that mimic the charge surface of double-stranded B-DNA
Nature Chemistry (2018)
-
Dynamic control of chirality and self-assembly of double-stranded helicates with light
Nature Chemistry (2017)
-
Syntheses and crystal structures of two self-assembled dizinc(II) helicates with novel hydrazone linked polypyridyl ligands
Research on Chemical Intermediates (2017)
-
Micelle formation induced by photo-Claisen rearrangement of poly(4-allyloxystyrene)-block-polystyrene
Colloid and Polymer Science (2009)
-
Synthesis of spherical particles by self-assembly of poly[2-(perfluorooctyl)ethyl acrylate-co-acrylic acid] in supercritical carbon dioxide
Colloid and Polymer Science (2008)