Abstract
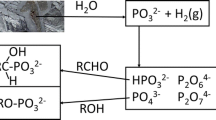
THE 1976 Mars Viking biology experiments were designed to detect life by observing the products of biochemical reactions. In the labelled-release (LR) experiments1–4, about 25 nmol of 14C-labelled gases evolved when regolith samples were moistened with nutrient solution. About 22% of the products reabsorbed upon second injection. As a biological test the LR results were positive, although the reabsorption was not readily explained. In the gas-exchange (GEX) experiments, up to 800 nmol of O2 gas was evolved when samples were humidified5,6, suggesting that the martian regolith might contain a strong chemical oxidant which caused the LR results. Several chemical models have been proposed7,8 but no self-consistent explanation of all of the observations has been achieved. Here we propose a chemical model for these biology experiments in which the reactants are an inorganic nitrate salt, which has been partly photolysed by ultraviolet light, and a sparingly soluble metal carbonate such as calcite. The model reproduces the main effects seen, indicating that nitrates are present in the martian regolith as well as calcite (or some other carbonate with similar solubility).
Similar content being viewed by others
References
Klein, H. P. et al. Science 194, 99–105 (1976).
Levin, G. V. & Straat, P. A. Science 194, 1322–1329 (1976).
Levin, G. V. & Straat, P. A. J. geophys. Res. 82, 4663–4667 (1977).
Levin, G. V. & Straat, P. A. J. molec. Evol. 14, 167–183 (1979).
Oyama, V. I., Berdahl, B. J. & Carle, G. C. Nature 265, 110–114 (1977).
Oyama, V. I. & Berdahl, B. J. J. geophys. Res. 82, 4669–4676 (1977).
Klein, H. P. Rev. Geophys. Space Phys. 17, 1655–1662 (1979).
Klein, H. P. J. molec. Evol. 14, 161–165 (1979).
Levin, G. V. & Straat, P. A. J. molec. Evol. 14, 185–197 (1979).
Ponnamperuma, C., Shimoyama, A., Yamada, M., Hobo, T. & Pal, R. Science 197, 455–457 (1977).
Ballou, E. V., Wood, P. C., Wydeven, T., Lehwalt, M. E. & Mack, R. E. Nature 271, 644–645 (1978).
Oyama, V. I. & Berdahl, B. J. J. molec. Evol. 14, 199–210 (1979).
Huguenin, R. L., Miller, K. J. & Harwood, W. S. J. molec. Evol. 14, 103–132 (1979).
Levin, G. V. & Straat, P. A. Icarus 45, 494–516 (1981).
Banin, A. & Rishpon, J. J. molec. Evol. 14, 133–152 (1979).
Nussinov, M. D., Chernyak, Y. B. & Ettinger, J. L. Nature 274, 859–861 (1978).
Narayanswamy, K. L. Trans. Faraday Soc. 31, 1411–1412 (1935).
Allen, A. O. & Ghormley, J. A. J. chem. Phys. 15, 208–209 (1947).
Doigan, P. & Davis, T. W. J. phys. Chem. 56, 764–766 (1952).
Hennig, G., Lees, R. & Matheson, M. S. J. chem. Phys. 21, 664–668 (1953).
Chen, T. H. & Johnson, E. R. J. phys. Chem. 66, 2249–2253 (1962).
Yurmazova, T. A., Koval, L. N. & Serikov, L. V. Khimiya Vysokikh Energii 17, 151–155 (1983).
Papee, H. M. & Petriconi, G. L. Nature 204, 142–144 (1964).
Clark, B. C. & VanHart, D. C. Icarus 45, 370–378 (1981).
Toon, O. B., Pollack, J. B. & Sagen, C. Icarus 30, 663–696 (1977).
Yung, Y. L., Strobel, D. F., Kong, T. Y. & McElroy, M. B. Icarus 30, 26–41 (1977).
McElroy, M. B., Yung, Y. L. & Nier, A. O. Science 194, 70–72 (1976).
Plumb, R. C., Thivierge, R. F. & Xu, W. W. J. phys. Chem. 91, 6074–6076 (1987).
Kuhn, W. R. & Atreya, S. K. J. molec. Evol. 14, 57–64 (1979).
Bevington, P. R. Data Reduction and Error Analysis for the Physical Sciences Ch. 11 (McGraw-Hill, New York, 1969).
Brown, F. S. et al. Rev. Sci. Instr. 49, 139–182 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Plumb, R., Tantayanon, R., Libby, M. et al. Chemical model for Viking biology experiments: implications for the composition of the martian regolith. Nature 338, 633–635 (1989). https://doi.org/10.1038/338633a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/338633a0
- Springer Nature Limited
This article is cited by
-
The Sample Analysis at Mars Investigation and Instrument Suite
Space Science Reviews (2012)
-
Viking Biology Experiments: Lessons Learned and the Role of Ecology in Future Mars Life-Detection Experiments
Space Science Reviews (2008)
-
It's life...isn't it?
Nature (2004)