Abstract
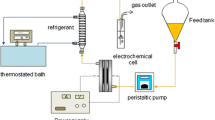
ONE of the major functions of an artificial kidney is the removal and excretion of nitrogenous waste products. A newer type of artificial kidney (REDY)1–3 converts the urea to ammonia by the action of encapsulated urease. For elimination, the toxic ammonia must then be adsorbed. This device uses a conventional type of dialyser with a recirculating regenerative dialysate system, which does not need large quantities of dialysis fluid. As the dialysis membrane will not filter bacteria, the system can be filled with a small volume of tap water. In practice, a multitude of unwanted waste materials are adsorbed on an activated carbon cartridge, and the urea is converted to ammonia which is then adsorbed on zirconium phosphate. The phosphate concentration of the dialysate is controlled with zirconium oxide; unfortunately the zirconium compounds are expensive, thus one of the major potential advantages of the system, low cost in use, is not achieved.
Similar content being viewed by others
References
Gordon, A., Greenbaum, M. A., Marantz, L. B., McArthur, J., and Maxwell, M. H., Trans. Amer. Soc. Artif. Int. Organs, 15, 347 (1969).
Gordon, A., Better, O. S., Greenbaum, M. A., Marantz, L. B., Gral, T., and Maxwell, M. H., Trans. Amer. Soc. Artif. Int. Organs, 17, 253 (1971).
Greenbaum, M. A., and Gordon, A., Dialysis and Transplantation, 1, 18 (1972).
Yao, S. J., Appleby, A. J., Geisel, A., Cash, jun., H. R., and Wolfson, jun., S. K., Nature, 224, 921 (1969).
Wolfson, jun., S. K., and Yao, S. J., in Proc. 7th Intersociety Energy Conversion Engineering Conf., 733 (American Chemical Society, 1972).
Yao, S. J., Appleby, A. J., and Wolfson, jun., S. K., Z. Phys. Chem. (Neue Folge) (in the press).
Giner, J., and Malachesky, P., Proc. Artif. Heart Program Conf., 839 (US Dept. Health, Education and Welfare, 1969).
Giner, J., Electrochim. Acta, 8, 857 (1963).
Giner, J., Electrochim. Acta, 9, 63 (1964).
Yao, S. J., and Wolfson, jun., S. K., Trans. Amer. Soc. Artif. Int. Organs, 18, 60 (1972).
Wolfson, jun., S. K., Yao, S. J., Geisel, A., and Cash, jun., H. R., Trans. Amer. Soc. Artif. Int. Organs, 16, 193 (1970).
Yao, S. J., Michuda, M., Markley, F., and Wolfson, jun., S. K., From Electrocatalysis to Fuel Cells (edit. by Sandstede, G.), 291 (University of Washington Press, 1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
YAO, S., WOLFSON, S., AHN, B. et al. Anodic Oxidation of Urea and an Electrochemical Approach to De-ureation. Nature 241, 471–472 (1973). https://doi.org/10.1038/241471a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/241471a0
- Springer Nature Limited
This article is cited by
-
Boosting urea electrooxidation on oxyanion-engineered nickel sites via inhibited water oxidation
Nature Communications (2023)
-
Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst
Nature Energy (2021)
-
Urea oxidation electrocatalysis on nickel hydroxide: the role of disorder
Journal of Solid State Electrochemistry (2021)
-
Electrocatalytic Oxidation of Glucose at Nickel Phosphate Nano/Micro Particles Modified Electrode
Electrocatalysis (2017)
-
Electrochemical treatment of aqueous solutions containing urea
Journal of Applied Electrochemistry (2009)