Abstract
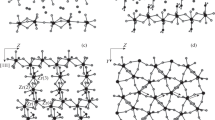
SPECIFIC heat measurements1–3 have shown that solid hydrogen sulphide undergoes a discontinuous transition at 103.5 K followed by a lambda transition which is completed at about 126.2 K. Similar transitions were found4 in solid deuterium sulphide at somewhat higher temperatures, 107.8 and 132.9 K respectively. The lowest solid phase of both substances is optically anisotropic4 whereas the two higher phases are isotropic. Dielectric constant measurements5–7 suggested that the orientation of the hydrogen sulphide molecules is ordered in the lowest solid phase and disordered in the two higher phases. This view gained further support from infrared8–11, Raman12,13 and nuclear magnetic resonance14,15 studies. No model has been put forward for the structure of the ordered phase; the investigators could not agree even on its symmetry. Up to now eight different point group symmetries have been proposed, the majority favouring tetragonal symmetry with eight molecules in the primitive unit cell.
Similar content being viewed by others
References
Clusius, K., Nachr. Ges. Wiss., Math. Phys. Kl. Fachgr. III, 171 (Göttingen, 1933).
Clusius, K., and Frank, A., Z. Phys. Chem., B, 34, 420 (1936).
Giauque, W. F., and Blue, R. W., J. Amer. Chem. Soc., 58, 831 (1936).
Kruis, A., and Clusius, K., Z. Phys. Chem., B, 38, 156 (1937).
Kemp, J. D., and Denison, G. H., J. Amer. Chem. Soc., 55, 251 (1933).
Smyth, P. C., and Hitchcock, C. S., J. Amer. Chem. Soc., 56, 1084 (1934).
Havriliak, S., Swenson, R. W., and Cole, R. H., J. Chem. Phys., 23, 134 (1955).
Lohman, J. B., Reding, F. P., and Hornig, D. F., J. Chem. Phys., 19, 252 (1951).
Reding, F. P., and Hornig, D. F., J. Chem. Phys., 27, 1024 (1957).
Anderson, A., and Walmsley, S. H., Mol. Phys., 9, 1 (1965).
Taimsalu, P., and Robinson, D. W., Spectrochim. Acta, 21, 1921 (1965).
Murphy, G. M., and Vance, J. E., J. Chem. Phys., 6, 426 (1938).
Miller, R. E., and Leroi, G. E., J. Chem. Phys., 49, 2789 (1968).
Alpert, N. L., Phys. Rev., 75, 398 (1949).
Look, D. C., Lowe, I. J., and Northby, J. A., J. Chem. Phys., 44, 3441 (1966).
Natta, G., Rend. R. Accad. Lincei, 11, 679, 749 (1930).
Natta, G., Nature, 127, 129 (1931).
Vegard, L., Nature, 126, 916 (1930).
Vegard, L., and Oserød, L. S., Avh. Norske Vidensk. Akad., I, Mat. Naturv. Kl., 7 (1942).
Vegard, L., Avh. Norske Vidensk. Akad., I, Mat. Naturv. Kl., 6 (1943).
Justi, E., and Nitka, H., Phys. Z., 37, 435 (1936).
Kitamura, N., Kashiwase, Y., Harada, J., and Honjo, G., Acta Cryst., 14, 687 (1961).
Kitamura, N., and Harada, J., J. Phys. Soc. Japan, 17, Suppl. B-II, 245 (1962).
Harada, J., and Kitamura, N., J. Phys. Soc. Japan, 19, 328 (1964).
Sándor, E., and Farrow, R. F. C., Nature, 213, 171 (1967).
Sándor, E., and Farrow, R. F. C., Nature, 215, 1265 (1967).
Busing, W. R., Martin, K. C., and Levy, H. A., Rep. No. ORNL-TM-30, Oak Ridge National Laboratory (1962).
Burrus, C. A., and Gordy, W., Phys. Rev., 92, 274 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SÁNDOR, E., OGUNADE, S. Structure and Phase Transition in Solid Hydrogen and Deuterium Sulphides. Nature 224, 905–907 (1969). https://doi.org/10.1038/224905b0
Received:
Issue Date:
DOI: https://doi.org/10.1038/224905b0
- Springer Nature Limited