Abstract
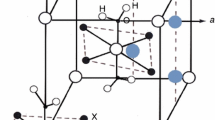
The effect of hydrate number on the structural changes, thermal properties, and ionic (molecular) mobility character in NH4ZrF5 ⋅ H2O, NH4ZrF5 ⋅ 0.75H2O crystal hydrates have been investigated by the methods of IR, Raman, nuclear magnetic resonance (NMR) (1H, 19F, including 19F MAS), and TG-DTA spectroscopy. Differences in crystal hydrate structures—anion structure, molecular state of water, and O–H⋅⋅⋅F, N–H⋅⋅⋅F hydrogen bond strengths—have been corroborated by IR and Raman spectroscopy data. Isotropic chemical shifts of magnetic inequivalent positions have been determined and attributed to crystal structures of the studied compounds by the method of 19F MAS NMR. It has been established that the removal of water molecules from NH4ZrF5 ⋅ H2O and NH4ZrF5 ⋅ 0.75H2O results in the transformation of chain or layered structures accompanied by the increase of the number of bridge bonds while retaining or increasing the dimensionality of the anion structural motif. According to the 1H NMR data, the NH\(_{4}^{ + }\) cation diffusion in NH4ZrF5 occurs only in the temperature range of 370–520 K.





Similar content being viewed by others
REFERENCES
V. Ya. Kavun and V. I. Sergienko, Diffusion Mobility and Ion Transport in Crystalline and Amorphous Fluorides of Elements of Group IV and Antimony (III) (Dal’nauka, Vladivostok, 2004) [in Russian].
V. Ya. Kavun, I. A. Tkachenko, N. A. Didenko, A. B. Slobodyuk, and V. I. Sergienko, Russ. J. Inorg. Chem. 55, 1179 (2010).
V. Ya. Kavun, V. I. Sergienko, B. N. Chernyshov, N. A. Didenko, N. G. Bakeeva, and L. N. Ignat’eva, Zh. Neorg. Khim. 36, 1004 (1991).
V. Gaument, C. Latouche, D. Avignant, and J. Dupuis, Solid State Ionics 74, 29 (1994).
A. V. Gerasimenko, K. A. Gaivoronskaya, A. B. Slobodyuk, and N. A. Didenko, Z. Anorg. Allg. Chem. 643, 1785 (2017).
N. A. Didenko, K. A. Gaivoronskaya, E. I. Voit, A. V. Gerasimenko, and V. Ya. Kavun, Russ. J. Inorg. Chem. 55, 1339 (2010).
E. I. Voit, N. A. Didenko, K. A. Gayvoronskaya, and A. V. Gerasimenko, Opt. Spectrosc. 121, 229 (2016).
K. A. Gaivoronskaya, A. V. Gerasimenko, N. A. Di-denko, A. V. Slobodyuk, and V. Ya. Kavun, Russ. J. Inorg. Chem. 58, 189 (2013).
M. M. Godneva and D. L. Motov, Chemistry Subgroups of Titanium. Sulfates, Fluorides, Fluorosulfates from Non-Aqueous Media (Nauka, Moscow, 2006) [in Russian].
I. V. Tananaev, L. S. Guzeeva, and K. I. Petrov, Izv. Akad. Nauk SSSR, Ser. Khim. 1, 103 (1968).
H. Hull and A. G. Turnbull, J. Inorg. Nucl. Chem. 29, 2903 (1967).
P. W. Smith, R. Stoessiger, and A. G. Turnbull, J. Chem. Soc. A, 3013 (1968).
E. I. Voit, N. V. Didenko, and K. A. Gaivoronskaya, Opt. Spectrosc. 124, 328 (2018).
B. V. Bukvetskii, A. V. Gerasimenko, and R. L. Davidovich, Koord. Khim. 17, 35 (1991).
A. Zalkin, D. Eimerl, and S. P. Velsko, Acta Crystallogr., C 44, 2050 (1988).
V. V. Tkachev, A. A. Udovenko, R. L. Davidovich, and L. O. Atovmyan, Koord. Khim. 17, 1635 (1991).
K. A. Gaivoronskaya, A. V. Gerasimenko, and N. A. Didenko, J. Struct. Chem. 59, 618 (2018).
A. V. Gerasimenko, V. Ya. Kavun, V. I. Sergienko, and T. F. Antokhina, Russ. J. Coord. Chem. 25, 562 (1999).
B. V. Bukvetskii, A. V. Gerasimenko, and R. L. Davidovich, Koord. Khim. 17, 35 (1991).
V. V. Tkachev, R. L. Davidovich, and L. O. Atovmyan, Koord. Khim. 17, 1485 (1991).
Y. Gao and J. Guery, J. Solid State Chem. 98, 11 (1992).
D. Avignant, I. Mansouri, R. Chevalier, and J. C. Cousseins, J. Solid State Chem. 38, 121 (1981).
R. E. Youngman and S. Sen, Solid State NMR 27, 77 (2005).
E. P. Zeer, V. E. Zobov, and O. V. Falaleev, New Effects in NMR of Polycrystals (Nauka, Novosibirsk, 1991) [in Russian].
B. V. Bukvetskii, A. V. Gerasimenko, and R. L. Davidovich, Koord. Khim. 18, 576 (1992).
C. R. Ross II, M. R. Bauer, R. M. Nielson, and S. C. Abrahams, Acta Crystallogr. 58, 841 (2002).
A. V. Gerasimenko and R. L. Davidovich, Vestn. DVO RAN, No. 5, 17 (2006).
E. I. Voit, A. V. Voit, A. V. Gerasimenko, and V. I. Sergienko, Zh. Strukt. Khim. 41, 255 (2000).
A. Ben Ali, M. Body, M. Leblanc, and V. Maisonneuve, Solid State Sci. 13, 394 (2011).
ACKNOWLEDGMENTS
This work was performed with the financial support of the Ministry of Education and Science of the Russian Federation, Federal Agency for Scientific Organizations, project no. 0265-2018-0001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Marinin
Rights and permissions
About this article
Cite this article
Voit, E.I., Slobodyuk, A.B. & Didenko, N.A. A Study of the Composition, Ionic and Molecular Mobility, and Thermal Properties of Ammonium Pentafluoridozirconate Hydrates. Opt. Spectrosc. 125, 888–897 (2018). https://doi.org/10.1134/S0030400X19020243
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X19020243