Abstract
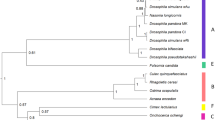
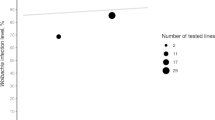
Wolbachia are endosymbiotic bacteria, widespread in terrestrial Arthropods. They are mainly transmitted vertically, from mothers to offspring and induce various alterations of their hosts’ sexuality and reproduction, the most commonly reported phenomenon being Cytoplasmic Incompatibility (CI), observed in Drosophila melanogaster and D. simulans. Basically, CI results in a more or less intense embryonic mortality, occurring in crosses between males infected by Wolbachia and uninfected females. In D. simulans, Wolbachia and CI were observed in 1986. Since then, this host species has become a model system for investigating the polymorphism of Wolbachia infections and CI. In this review we describe the different Wolbachia infections currently known to occur in D. melanogaster and D. simulans. The two species are highly contrasting with regard to symbiotic diversity: while five Wolbachia variants have been described in D. simulans natural populations, D. melanogaster seems to harbor one Wolbachia variant only. Another marked difference between these two Drosophila species is their permissiveness with regard to CI, which seems to be fully expressed in D. simulans but partially or totally repressed in D. melanogaster, demonstrating the involvement of host factors in the control of CI levels. The potential of the two host species regarding the understanding of CI and its evolution is also discussed.
Similar content being viewed by others
References
Ballard, J.W.O., 2000a. When one is not enough: introgression of mitochondrial DNA in Drosophila. Mol. Biol. Evol. 17: 1126–1130.
Ballard, J.W.O., 2000b. Comparative genomics of mitochondrial DNA in Drosophila simulans. J. Mol. Evol. 51: 64–75.
Ballard, J.W.O., J. Hatzidakis, T.L. Karr & M. Karr, 1996. Reduced variation in Drosophila simulans mitochondrial DNA. Genetics 144: 1519–1528.
Bandi, C., T.J.C. Anderson, C. Genchi & M.L. Blaxter, 1998. Phylogeny of Wolbachia bacteria in filarial nematodes. Proc. R. Soc. Lond. B 265: 2407–2413.
Bandi, C., A.J. Trees & N.W. Brattig, 2001. Wolbachia in filerial nematodes: Evolutionnary aspects and implication for the pathogenesis and treatment of filarial diseases. Vet. Parasitol. 98: 215–238.
Binnington, K.C. & A.A. Hoffmann, 1989. Wolbachia-like organisms and cytoplasmic incompatibility in Drosophila simulans. J. Invert. Pathol. 54: 344–352.
Bourtzis, K., A. Nirgianaki, P. Onyango & C. Savakis, 1994. A prokaryotic dnaA sequence in Drosophila melanogaster: Wolbachia infection and cytoplasmic incompatibility among laboratory strains. Inset. Mol. Biol. 3: 131–142.
Bourtzis, K., A. Nirgianaki, G. Markakis & C. Savakis, 1996. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144: 1063–1073.
Bourtzis, K., S.L. Dobson, H.R. Braig & S.L. O'Neill, 1998. Rescuing Wolbachia have been overlooked. Nature 391: 852–853.
Boyle, L., S.L. O'Neill, H.M. Robertson & T.L. Karr, 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260: 1796–1799.
Braig, H.R., H. Guzman, R.B. Tesh & S.L. O'Neill, 1994. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature 367: 453–455.
Bressac, C. & F. Rousset, 1993. The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J. Invert. Pathol. 63: 226–230.
Callaini, G., M.G. Riparbelli, R. Giordano & R. Dallai, 1996. Mitotic defects associated with cytoplasmic incompatibility in Drosophila simulans. J. Invert. Pathol. 67: 55–64.
Callaini, G., R. Dallai & M.G. Ripardelli, 1997. Wolbachia-induced delay of paternal chromatin condensation does prevent maternal chromosomes from entering anaphase in incompatible crosses in Drosophila simulans. J. Cell Biol. 110: 271–280.
Casiraghi, M., T.J.C. Anderson, C. Bandi, C. Bazzochi & C. Genchi, 2001. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology 122: 93–103.
Charlat, S. & H. Merçot, 2001. Wolbachia, mitochondria and sterility. Trends Ecol. Evol. 16: 431–432.
Charlat, S., K. Bourtzis & H. Merçot, 2001. Wolbachia-induced cytoplasmic incompatibility, pp. 621–644 in Symbiosis: mechanisms and model systems, edited by J. Seckbach. Kluwer Academic Publishers, Dordrecht.
Charlat, S., A. Nirgianaki, K. Bourtzis & H. Merçot, 2002. Evolution of Wolbachia-induced cytoplasmic incompatibility in Drosophila simulans and D. sechellia. Evolution 56: 1735–1742.
Charlat, S., P. Bonnavion & H. Merçot, 2003. Wolbachia segregation dynamics and levels of cytoplasmic incompatibility in Drosophila sechellia. Heredity 90: 157–161.
Charlat, S., L. Le Chat & H. Merçot, 2003. Characterization of non-cytoplasmic incompatibility inducing Wolbachia in two continental African populations of Drosophila simulans. Heredity 90: 49–55.
Clancy, D. & A.A Hoffmann, 1997. Behavior of Wolbachia endosymbionts from Drosophila simulans in Drosophila serrata, a novel host. Am. Nat. 149: 975–988.
Dobson, S.L., K. Bourtzis, H.R. Braig, B.F. Jones, W. Zhou, F. Rousset & S.L. O'Neill, 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29: 153–160.
Erickson, J. & A.B. Acton, 1969. Spermatocyte granules in Drosophila melanogaster. Can. J. Genet. Cytol. 11: 153–168.
Ghelelovitch, S., 1952. Sur le déterminisme génétique de la sterilité dans le croisement entre differentes souches de Culex autogenicus Roubaud. C. R. Acad. Sci. Paris 24: 2386–2388.
Giordano, R., S.L. O'Neill & H.M. Robertson, 1995. Wolbachia infections and the expression of cytoplasmic incompatibility in Drosophila sechellia and D. mauritiana. Genetics 140: 1307–1317.
Hertig, M., 1936. The rickettsia Wolbachia pipientis (gen. et sp. n.) and associated inclusions of the mosquitos, Culex pipiens. Parasitology 28: 453–486.
Hertig, M. & S.B. Wolbach, 1924. Studies on rickettsia-like microorganisms in insects. J. Med. Res. 44: 329–374.
Hey, J. & R.M. Kliman, 1993. Population genetics and phylogenetics of DNA sequence variation at multiple loci within the Drosophila melanogaster species complex. Mol. Biol. Evol. 10: 804–822.
Hoffmann, A.A., 1988. Partial cytoplasmic incompatibility between two australian populations of Drosophila melanogaster. Entomol. Exp. Appl. 48: 61–67.
Hoffmann, A.A. & M. Turelli, 1988. Unidirectional incompatibility in Drosophila simulans: Inheritance, geographic variation and fitness effects. Genetics 119: 435–444.
Hoffmann, A.A. & M. Turelli, 1997. Cytoplasmic incompatibility in insects, pp. 42–80 in Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, edited by S.L. O'Neill, A.A. Hoffmann and J.H. Werren. Oxford University Press, Oxford.
Hoffmann, A.A., M. Turelli & G.M. Simmons, 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40: 692–701.
Hoffmann, A.A., M. Turelli & L.G. Harshman, 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126: 933–948.
Hoffmann, A.A., D.J. Clancy & E. Merton, 1994. Cytoplasmic incompatibility in australian populations of Drosophila melanogaster. Genetics 136: 993–999.
Hoffmann, A.A., D.J. Clancy & J. Ducan, 1996. Naturally occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibilty. Heredity 76: 1–8.
Hoffmann, A.A., M. Hercus & H. Dagher, 1998. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in populations of Drosophila melanogaster. Genetics 148: 221–231.
Holden, P.R., P. Jones & J.F.Y. Brookfield, 1993. Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet. Res. Camb. 62: 23–29.
Hurst, G.D. & F.M. Jiggins, 2000. Male-killing bacteria in insects: mechanisms, incidence and implications. Emerging Infect. Dis. 6: 329–336.
James, A.C. & J.W.O. Ballard, 2000. The expression of cytoplasmic incompatibility and its impact on population frequencies and the distribution of Wolbachia strains in Drosophila simulans. Evolution 54: 1661–1672.
James, A.C., M.D. Dean, M.E. McMahon & J.W.O. Ballard, 2002. Dynamics of double and single Wolbachia infections in Drosophila simulans from New Caledonia. Heredity 88: 182–189.
King, R.C., 1970. Ovarian Development in Drosophila melanogaster. Academic Press, New York, 227 pp.
King, R.C. & R.P. Mills, 1962. Oogenesis in adult Drosophila melanogaster. XI Studies of some organelles of the nutrient stream in egg chambers of D. melanogaster and D. willistoni. Growth 21: 235–253.
Kose, H. & T.L. Karr, 1995. Organization of Wolbachia pipientis in the Drosophila fertilized egg and embryo revealed by an anti-Wolbachia monoclonal antibody. Mech. Dev. 51: 275–288.
Lachaise, D., M. Harry, M. Solignac, F. Lemeunier, V. Bénassi & M-L. Carriou. 2000. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from Sao Tomé. Proc. R. Soc. Lond. B 267: 1487–1495.
Lassy, C.W. & T.L. Karr, 1996. Cytological analysis of fertilization and early embryonic development in incompatible crosses of Drosophila simulans. Mech. Dev. 57: 47–58.
Lo, N., M. Casiraghi, E. Salati, C. Bazzocchi & C. Bandi, 2002. How many Wolbachia supergroups exist? Mol. Biol. Evol. 19: 341–346.
Merçot, H. & D. Poinsot, 1998a. Wolbachia transmission in a naturally bi-infected Drosophila simulans strain from New Caledonia. Entomol. Exp. Appl. 86: 97–103.
Merçot, H. & D. Poinsot, 1998b. Rescuing Wolbachia have been overlooked and discovered on Mount Kilimanjaro. Nature 391: 853.
Merçot, H., B. Llorente, M. Jacques, A. Atlan & C. Montchamp-Moreau, 1995. Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics 141: 1015–1023.
Min, K.T. & S. Benzer, 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94: 10792–10796.
Montchamp-Moreau, C., J-F. Ferveur & M. Jacques, 1991. Geographic distribution and inheritance of three cytoplasmic incompatibility types in Drosophila simulans. Genetics 129: 399–407.
Nigro, L., 1991. The effect of heteroplasmy on cytoplasmic incompatibility in transplasmic lines of Drosophila simulans showing a complete replacement of the mitochondrial DNA. Heredity 66: 41–45.
Olsen, K., K.T. Reynolds & A.A. Hoffmann, 2001. A field cage test of the effects of the endosymbiont Wolbachia on Drosophila melanogaster. Heredity 86: 731–737.
O'Neill, S.L. & T.L. Karr, 1990. Bi-directional incompatibility between conspecific populations of Drosophila simulans. Nature 348: 178–180.
O'Neill, S.L., R. Giordano, A.M.E. Colbert, T.L. Karr & H.M. Robertson, 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89: 2699–2702.
O'Neill, S.L., A.A. Hoffmann & J.H. Werren (eds), 1997. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press, Oxford, 226 pp.
Peacock, W.J. & J. Erickson, 1964. An indicator of polarity in the spermatocyte? Drosophila Inform. Serv. 39: 107–108.
Poinsot, D. & H. Merçot, 1999. Wolbachia can rescue from cytoplasmic incompatibility while being unable to induce it, pp. 221-234 in From Symbiosis to Eukaryotism-ENDOCYTOBIOLOGY VII, edited by E. Wagner et al. Universities of Geneva and Freiburg im Breisgau.
Poinsot, D. & H. Merçot, 2001. Wolbachia injection from usual to naïve host in Drosophila simulans (Diptera: Drosophilidae). Eur. J. Entomol. 98: 25–30.
Poinsot, D., K. Bourtzis, G. Markakis, C. Savakis & H. Merçot, 1998. Injection of a Wolbachia from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150: 227–237.
Poinsot, D., M. Montchamp-Moreau & H. Merçot, 2000. Wolbachia segregation rate in Drosophila simulans bi-infected cytoplasmic lineages. Heredity 85: 191–198.
Poinsot, D., S. Charlat & H. Merçot, 2003. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: confrounting the models to the facts. BioEssays 25: 259–265.
Reynolds, K.T. & A.A. Hoffmann, 2002. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. Camb. 80: 79–87.
Riegler, M., S. Charlat, C. Stauffer & H. Merçot, 2003. Wolbachia transfer from a true fruit fly into the real fruit fly: investigating the outcomes of host/symbiont co-evolution (submitted).
Roux, V. & D. Raoult, 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146: 385–396.
Rousset, F., 1993. Les facteurs déterminant la distribution des Wolbachia, bactéries endosymbiotiques des arthropodes. Thèse de Doctorat de l'Université Paris Sud Orsay, 114 pp.
Rousset, F. & E. de Stordeur, 1994. Properties of Drosophila simulans strains experimentally infected by different clones of the bacterium Wolbachia. Heredity 71: 325–331.
Rousset, F. & M. Solignac, 1995. Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proc. Natl. Acad. Sci. USA 92: 6389–6393.
Rousset, F., D. Vautrin & M. Solignac, 1992. Molecular identification of Wolbachia, the agent of cytoplasmic incompatibility in Drosophila simulans and variability in relation with host mitochondrial types. Proc. R. Soc. Lond. B 247: 163–168.
Rousset, F., H.R. Braig & S.L. O'Neill, 1999. A stable triple Wolbachia infection in Drosophila with nearly additive incompatibility effects. Heredity 82: 620–627.
Solignac, M. & M. Monnerot, 1986. Race formation, speciation, and introgression within Drosophila simulans, D. mauritiana, and D. sechellia inferred from mitochondrial DNA analysis. Evolution 40: 531–539.
Solignac, M., D. Vautrin & F. Rousset, 1994. Widespread occurrence of the proteobacteria Wolbachia and partial cytoplasmic incompatibility in Drosophila melanogaster. C. R. Acad. Sci. Paris 317: 461–470.
Stouthamer, R., J.A. Breeuwer & G.D. Hurst, 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Ann. Rev. Microbiol. 53: 71–102.
Szollosi, D. & A. Debec, 1980. Presence of Rickettsias in haploid Drosophila melanogaster cell lines. Biol. Cell. 38: 129–134.
Tram, U. & W. Sullivan, 2002. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296: 1124–1126.
Turelli, M., 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48: 1500–1513.
Turelli, M. & A.A. Hoffmann, 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353: 440–442.
Turelli, M. & A.A. Hoffmann, 1995. Cytoplasmic incompatibility in Drosophila simulans: Dynamics and parameter estimates from natural populations. Genetics 140: 1319–1338.
Turelli, M., A.A. Hoffmann & S.W. McKechnie, 1992. Dynamics of cytoplasmic incompatibility and mtDNA variation in Drosophila simulans populations. Genetics 132: 713–723.
Ullmann, S.L., 1965. Epsilon granules in Drosophila pole cells and oocytes. J. Embryol. Exptl. Morphol. 13: 73–81.
Van Meer, M.M.M. & R. Stouthamer, 1999. Cross-order transfer of Wolbachia from Muscidiforax uniraptor (Hymenoptera: Pteromaldae) to Drosophila simulans (Diptera: Drosophilidae). Heredity 82: 163–169.
Vandekerckhove, T.T.M., S. Watteyne, A. Willems, J.G. Swing, J. Mertens & M. Gillis, 1999. Phylogenetic analysis of the 16S rRNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda: Collembola) and its implications for wolbachial taxonomy. FEMS Microbiol. Lett. 180: 279–286.
Weeks, A.R., K.T. Reynolds & A.A. Hoffmann, 2002. Wolbachia dynamics and host effects: What has (and has not) been demonstrated? Trends Ecol. Evol. 17: 257–262.
Werren, J.H., 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42: 587–609.
Werren, J.H., W. Zhang & L.R. Guo., 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. B 261: 55–63.
Wolstenholme, D.R., 1965. A DNA and RNA-containing cytoplasmic body in Drosophila melanogaster and its relation to flies. Genetics 52: 949–975.
Yanders, A.F., J.G. Brewen, W.J. Peackock & D.J. Goodchild, 1968. Meiotic drive and visible polarity in Drosophila spermatocytes. Genetics 59: 245–253.
Yen, J.H. & A.R. Barr, 1971. New hypothesis on the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232: 657–658.
Yen, J.H. & A.R. Barr, 1973. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invert. Pathol. 22: 242–250.
Zhou, W.G., F. Rousset & S.L. O'Neill, 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265: 509–515.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Merçot, H., Charlat, S. Wolbachia Infections in Drosophila Melanogaster and D. Simulans: Polymorphism and Levels of Cytoplasmic Incompatibility. Genetica 120, 51–59 (2004). https://doi.org/10.1023/B:GENE.0000017629.31383.8f
Issue Date:
DOI: https://doi.org/10.1023/B:GENE.0000017629.31383.8f