Abstract
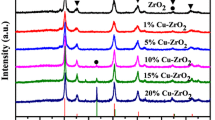
Fe-ZrO2 and Cu-ZrO2 xerogels were prepared by a sol-gel method. The effect of the hydrolysis catalyst during the gelation step, namely H2SO4 or NH4OH, on the properties of the resulting materials was investigated by XRD, BET, TGA/DTA, TPD of ammonia, FTIR, and TPR. Fe-ZrO2 and Cu-ZrO2 xerogels, with sulfuric acid introduced as the hydrolysis catalyst, mainly crystallyzed in the tetragonal phase and exhibited larger surface area and acid amount than those obtained with NH4OH. Ammonia TPD shows that copper promoted sulfated zirconia is the most acidic material. TGA and FTIR reveal that under oxidizing conditions sulfated zirconia promoted with iron and copper retains more sulfate species than unpromoted sulfated zirconia. Regardless of the hydrolysis catalyst employed, copper promoted catalysts calcined at 600°C, contain a large fraction of copper oxide specieseasily reduced at low temperatures. These copper oxide species are believed to have different environment and interactions with the surface oxygen vacancies of the zirconia support. A FeO-like phase appears to be the most probable one after reduction of Fe-ZrO2 catalysts prepared with NH4OH as the hydrolysis catalyst. The formation of Fe° species may be hindered by the high dispersion and interaction of Fe2+ ions with the zirconia support. On the other hand, the reduction peaks of iron oxide and sulfate species exhibit a considerable overlap in the TPR profiles of sulfated Fe-ZrO2 samples. Hence, the nature of the supported phase in the latter samples is rather uncertain.
Similar content being viewed by others
References
R. Srinivasan, T.R. Watkins, C.R. Hubbard, and B. Davis, Chem. Mat. 7, 725 (1995).
R.A. Comelli, C.V. Vera, and J.M. Parera, J. Catal. 151, 96 (1995).
M. Wakif, J. Bachelier, O. Saur, and J.C. Lavalley, J. Mol. Catal. 72, 127 (1992).
C. Morterra, E. Giamello, G. Cerrato, G. Centi, and P. Perathoner, J. Catal. 179, 111 (1998).
Y. Okamoto, H. Gotoh, T. Tanaka, and S. Yoshida, J. Chem. Soc. Faraday Trans. 93, 3879 (1997).
G. Centi, G. Cerrato, S. Dángelo, U. Finardi, E. Giamello, C. Morterra, and S. Perathoner, Catal. Today 27, 265 (1996).
L.A. Boot, A.J. van Dillen, J.W. Geus, and F.R. van Buren, J. Catal. 163, 186 (1996).
K. Chen, Y. Fan, Z. Hu, and Q. Yan, Catal. Lett. 36, 139 (1996).
R.A. Koppel, C. Stoker, and A. Baiker, J. Catal. 179, 515 (1998).
G. Fetter, P. Bosch, T. López, and R. Gómez, in Actas Simposio Iberoamericano de Catálisis (Armonia Impresores, Bucaramanga, 1998), p. 2231.
R.D. Gonzalez, Bing-hui Li, R. Gómez, and T. López, in Actas XIV Simposio Iberoamericano de Catálisis (Universidad de Concepción, Chile, 1994), p. 1623.
A. Calafat, Preparation of Catalysts VII (Elsevier 118, Amsterdam, 1998), p. 837.
H. Armendariz, B. Coq, D. Tichit, R. Dutartre, and F. Figueras, J. Catal. 173, 345 (1998).
R. Zhou, T. Yu, X. Jiang, F. Chen, and X. Zheng, Appl. Surf. Sci. 148, 263 (1999).
J.F. Collins and J.F. Ferguson, J. Chem. Soc. 4, 4 (1968).
F. Lónyi and J. Valyon, J. Thermal Anal. 46, 211 (1996).
R. Srinivisan, R.A. Keogh, D.R. Milburn, and B.H. Davis, J. Catal. 153, 123 (1995).
P.D.L. Mercera, J.G. van Ommen, E.B.M. Doesburg, A.J. Burggraaf, and J.R.H. Ross, Appl. Catal. 78, 79 (1991).
P. Berteau and B. Delmon, Catal. Today 5, 121 (1989).
G. Cerrato, F. Pinna, and M. Signoretto, J. Catal. 157, 109 (1995).
W. Dow, Y. Wang, and R. Huang, J. Catal. 160, 155 (1996).
W. Dow and R. Huang, J. Catal. 160, 171 (1996).
F. Figueras, B. Coq, E. Ensuque, D. Tachon, and G. Delahay, Catalysis Today 42, 117 (1998).
E. Guglielminotti, J. Phys. Chem. 98, 4884 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Castro, L., Reyes, P. & de Correa, C.M. Synthesis and Characterization of Sol-Gel Cu-ZrO2 and Fe-ZrO2 Catalysts. Journal of Sol-Gel Science and Technology 25, 159–168 (2002). https://doi.org/10.1023/A:1019920531309
Issue Date:
DOI: https://doi.org/10.1023/A:1019920531309