Abstract
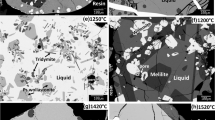
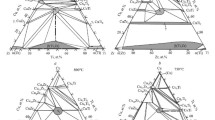
Phase relations in the system Cu-La-O at 1200 K have been determined by equilibrating samples of different average composition at 1200 K, and phase analysis of quenched samples using optical microscopy, XRD, SEM and EDX. The equilibration experiments were conducted in evacuated ampoules, and under flowing inert gas and pure oxygen. There is only one stable binary oxide La2O3 along the binary La-O, and two oxides Cu2O and CuO along the binary Cu-O. The Cu-La alloys were found to be in equilibrium with La2O3. Two ternary oxides CuLaO2 and CuLa2O4+δ were found to be stable. The value of δ varies from close to zero at the dissociation partial pressure of oxygen to 0.12 at 0.1 MPa. The ternary oxide CuLaO2, with copper in monovalent state, coexisted with Cu, Cu2O, La2O3, and/or CuLa2O4+δ in different phase fields. The compound CuLa2O4+δ, with copper in divalent state, equilibrated with Cu2O, CuO, CuLaO2, La2O3, and/or O2 gas under different conditions at 1200 K. Thermodynamic properties of the ternary oxides were determined using three solid-state cells based on yttria-stabilized zirconia as the electrolyte in the temperature range from 875 K to 1250 K. The cells essentially measure the oxygen chemical potential in the three-phase fields, Cu + La2O3 + CuLaO2, Cu2O + CuLaO2 + CuLa2O4 and La2O3 + CuLaO2 + CuLa2O4. Although measurements on two cells were sufficient for deriving thermodynamic properties of the two ternary oxides, the third cell was used for independent verification of the derived data. The Gibbs energy of formation of the ternary oxides from their component binary oxides can be represented as a function of temperature by the equations:
Similar content being viewed by others
References
B. VON GRANDE, HK. MÜLLER BUSCHBAUM and M. SCHWEIZER, Z. Anorg. Allg. Chem. 428 (1977) 120.
E. J. OPILA and H. L. TULLER, Mater. Res. Soc. Symp. Proc. 209 (1991) 867.
S. NISHIYAMA, N. KIEDA, K. SHINOZAKI, M. KATO and N. MIZUTANI, J. Ceram. Soc. Jpn. Inter. Ed. 97 (1989) 1114.
N. KIEDA, S. NISHIYAMA, K. SHINOZAKI and N. MIZUTANI, Solid State Ionics 49 (1991) 85.
C. CHAILLOUT, S. W. CHEONG, Z. FISH, M. S. LEHMANN, M. MAREZIO, B. MOROSIN and J. E. SCHIRBER, Physica C 158 (1989) 183.
B. O. WELLS, Y. S. LEE, M. A. KASTNER, R. J. CHRISTIANSON, R. J. BIRGENEAU, K. YAMADA, Y. ENDOH and G. SHIRANE, Science 277 (1997) 1067.
B. O. WELLS, R. J. BIRGENEAU, F. C. CHOU, Y. ENDOH, D. C. JOHNSTON, M. A. KASTNER, Y. S. LEE, G. SHIRANE, J. M. TRANQUADA and K. YAMADA, Z. Phys. B 100 (1996) 535.
J. D. JORGENSON, B. DABROWSKI, S. PEI, D. G. HINK, L. SODERHOLM, B. MOROSIN, J. E. SCHIRBER, E. L. VENTURINI and D. S. GINLEY, Phys. Rev. B. 38 (1988) 11 337.
P. G. RADAELLI, J. D. JORGENSON, R. KLEB, B. A. HUNTER, F. C. CHOU and D. C. JOHNSTON, ibid. 49 (1994) 6239.
YU. D. TRETYAKOV, A. R. KAUL and N. V. MAKUKHIN, J. Solid State Chem. 17 (1976) 183.
M. S. CHANDRASEKHARAIAH, M. D. KARKHANAVALA and O. M. SREEDHARAN, High Temp. Sci. 11 (1979) 65.
A. N. PETROV, A. YU. ZUEV and V. A. CHEREPANOV, Russ. J. Phys. Chem. 62 (1988) 1613.
J. BULARZIK, A. NAVROTSKY, J. DICARLO, J. BRINGLEY, B. SCOTT and S. TRAIL, J. Solid State Chem. 93 (1991) 418.
K. SUN, J. H. CHO, F. C. CHOU, W. C. LEE, L. L. MILLER and D. C. JOHNSTON, Phys. Rev. B. 43 (1991) 239.
R. J. CAVA, T. SIEGRIEST, B. HESSEN, J. J. KRAJEWSKI, W. F. PECK, JR., B. BATTLOGG, H. TAKAGI, J. V. WASZCZAK and L. F. SCNEEMEYER, J. Solid State Chem. 94 (1991) 170.
R. NORRESTAM, M. NYGREN and J.-O. BOVIN, Angew. Chem. Int. Ed. Engl. 30 (1991) 864.
J. FOULTIER, P. FABRY and M. KLEITZ, J. Electrochem. Soc. 123 (1976) 204.
K. T. JACOB and T. MATHEWS, Ind. J. Tech. 28 (1990) 413.
K. T. JACOB and K. P. JAYADEVAN, J. Mater. Chem. 7 (1997) 2407.
Idem., Mater. Sci. Eng. B 52 (1998) 134.
Idem., Chem. Mater. 12 (2000) 1779.
K. T. JACOB, G. M. KALE, and G. N. K. IYENGAR, Metall. Trans. B 20B (1989) 679.
K. T. JACOB and J. H. E. JEFFES, Trans. Inst. Min. Metall., Sec. C 80 (1971) C181.
Z. G. LI, H. H. FENG, Z. Y. YANG, A. HAMED, S. T. TING and P. H. HOR, Phys. Rev. Lett. 77 (1996) 5413.
D. C. JOHNSON, J. P. STOKES, D. P. GOSHORN and J. T. LEWANDOWSKI, Phys. Rev. B 36 (1987) 4007.
A. WALTIAUX, J. C. PARK, J. C. GRENIER and M. POUCHARD, C. R. Acad Sci. 310 (1990) 1047.
J. C. GRENIER, F. ARROUY, J. P. LOCQUET, C. MONROUX, M. POUCHARD, A. VILLESUZANNE and A. WALTIAUX, “Phase Separation in Cuprate Superconductors,” edited by E. Sigmund and K. A. Muller (Springer-Verlag, Berlin, 1994) p. 236.
S. N. RUDDLESDEN and P. POPPER, Acta Crystallogr. 11 (1958) 54.
R. J. CAVA, H. W. ZANDBERGEN, A. P. RAMIREZ, H. TAKAGI, C. T. CHEN, J. J. KRAJEWSKI, W. F. PECK JR., J. V. WASZCZAK, G. MEIGS, R. S. ROTH and L. F. SCNEEMEYER, J. Solid State Chem. 104 (1993) 437.
K. T. JACOB and J. H. E. JEFFES, Trans. Inst. Min. Metall., Sec. C 80 (1971) C32.
K. T. JACOB and C. B. ALCOCK, J. Amer. Ceram. Soc. 8 (1975) 192.
K. P. JAYADEVAN and K. T. JACOB, High Temp. Mater. Process. 19 (2000) 389.
L. B. PANKRATZ, Thermodynamic Properties of Elements and Oxides, U. S. Department of the Interior, Bureau of Mines Bull. 672, 1982, p. 214.
Z. DU, Y. XU and W. ZHANG, J. Alloys and Compds. 289 (1999) 88. Received 14 February and accepted 11 December 2001 1620
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacob, K.T., Jayadevan, K.P. Phase relations in the system Cu-La-O and thermodynamic properties of CuLaO2 and CuLa2O4 . Journal of Materials Science 37, 1611–1620 (2002). https://doi.org/10.1023/A:1014957910889
Issue Date:
DOI: https://doi.org/10.1023/A:1014957910889