Abstract
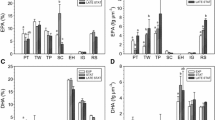
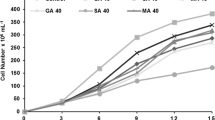
Three species of microalgae able to produce eicosapentaenoic acid(EPA) were collected from brackish and sea water around Japan. The species were identified as Navicula saprophila, Rhodomonassalina and Nitzschia sp. EPA as a proportion of total fatty acids increased in the presence of acetic acid for Rhodomonas salina and Nitzschia sp. However, Navicula saprophila displayed the greatest productivity of EPA and the EPA content of its biomass was enhanced under mixotrophic conditions in the presence of acetic acid.
Similar content being viewed by others
References
Bajpai P, Bajpai PK (1993) Eicosapentaenoic acid (EPA) production from microorganisms: a review. J. Biotech. 30: 161–183.
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917.
Chrismadha, Borowitzka MA (1994) Effect of cell density and irradiance on growth, proximate composition and eicosapentaenoic acid production of Phaeodactylum tricornutumgrown in a tubular photobioreactor. J. appl. Phycol. 6: 67–74.
Cohen Z, Vonshak A, Richmond A (1988) Effect of environmental conditions on fatty acid composition of the red alga Porphyridium cruentum: Correlation to growth rate. J. Phycol. 24: 328–332.
Dunstan GA, Volkman JK, Barrett SM, Leroi J, Jeffrey SW (1994) Essential polyunsaturated fatty acids from 14 species of diatom. Phytochemistry 35: 155–161.
Endo H, Hosoya H, Koibuchi T (1977) Growth yields of Chlorella regularisin dark heterotrophic continuous cultures using acetate. J. Ferment. Technol. 55: 369–379.
Endo H, Nakajima K, Chino R (1974) Growth characteristics and cellular components of Chlorella regularis, heterotrophic fast growing strain. Agr. biol. Chem. 38: 9–18.
Greenspan P, Mayer EP, Fowler SD (1985) Nile Red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100: 965–973.
Hill DRA (1991) A revised circumscription of Cryptomonasbased on examination of Australian strains. Phycologia 30: 170–188.
Hill DRA, Wetherbee R (1989) A reappraisal of the genus Rhodomonas. Phycologia 28: 143–158.
Krammer K, Lange-Bertalot H(1988) Bacillariophyceae 2. In Ettl H, Heyning H, Mollenhauer D (eds), Süsswasserflora von Mitteleuropa. Gustav Fischer, Stuttgart: 110–117.
Lange-Bertalot H, Bonik K (1976) Massenentwicklung bisher seltener und unbekannter Diatomeen als Indikator für starke Abwasserbelastung in Flüssen. Arch.Hydrobiol., Suppl. 49: 303–332.
Ogawa T, Aiba S (1981) Bioenergetic analysis ofmixotrophic growth in Chlorella vulgarisand Scenedesmus acutus. Biotechnol. Bioengng. 23: 1121–1132.
Qiang H, Zhengyu H, Cohen Z, Richmond A (1997) Enhancement of EPA and gammalinolenic acid production by manipulating algal density of outdoor cultures of Monodus subterraneusand Spirulina platensis. Eur. J. Phycol. 32: 81–86.
Samejima H, Myers J (1958) On the heterotrophic growth of Chlorella pyrenoidosa. J. gen. Microbiol. 18: 107–117.
Sanchéz PJA (1994) N–3 Polyunsaturated fatty acid productivity of themarine microalgal Isochrysis galbana. Growth conditions and phenotypic selection. J. appl. Phycol. 6: 475–478.
Seto A, Wang HL, Hesseltine CW (1984) Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. JAOCS 61: 892–894.
Tan CK and Johns MR (1996) Screening of diatom for heterotrophic eicosapentaenoic acid production. J. appl. Phycol. 8: 59–64.
Watanabe M, Nozaki H (1994) NIES-Collection,List of strains. National Institute for Environmental Studies, Environment Agency, Japan.
Yongmanitchai W, Ward OP (1989) Omega-3 fatty acids: Alternative sources of production. Process Biochem. August: 117–125.
Yongmanitchai W, Ward OP (1994a) Growth of and omega-3 fatty acid production by Phaeodactylum tricornutumunder different culture conditions. Appl. envir. Microbiol. 57: 419–425.
Yongmanitchai W, Ward OP (1991b) Screening of algae for potential alternative source of eicosapentaenoic acid. Phytochemistry 30: 2963–2967.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kitano, M., Matsukawa, R. & Karube, I. Changes in eicosapentaenoic acid content of Navicula saprophila, Rhodomonas salina and Nitzschia sp. under mixotrophic conditions. Journal of Applied Phycology 9, 559–563 (1997). https://doi.org/10.1023/A:1007908618017
Issue Date:
DOI: https://doi.org/10.1023/A:1007908618017