Abstract
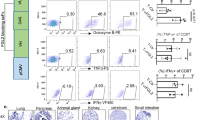
T cells are attractive for delivering therapy to brain tumor, especially disseminated micro-tumor. However, to trigger effector function, tumor antigen must be re-presented to T cells, via major histocompatibility complex (MHC) proteins, at the tumor site. In normal brain, MHC+ antigen-presenting cells (APC) are rare, but abundant after gamma interferon (IFN-γ) injection. Here we studied tumor-bearing brains. IFN-γ (or buffer) was injected stereotactically into brains with established tumors from a panel of immunologically varied glioma cell lines, some expressing b-galactosidase as a micro-tumor marker. Four days later, cryostat sections were stained for tumor and MHC proteins. In phosphate-buffered saline-injected controls, class II MHC+ potential APC (microglia, macrophages) were seen only at (some) tumor sites. In rats that received IFN-γ, class II+ potential APC were widespread, including all actual and potential micro-tumor sites and all tumor-free areas. In the same slides, neither class I nor class II MHC antigen was detected in neural cells or most tumor cells. This MHC pattern favors indirect re-presentation of tumor antigen, by tumor-adjacent APC. The robust response to IFN-γ might also be exploited in other ways: activated microglia and macrophages can attack tumor directly, and class II+ APC may help mark micro-tumor sites.
Similar content being viewed by others
References
Lampson LA: Beyond inflammation: site directed immunotherapy. Immunol Today 19: 17–22, 1998
Lampson LA: Basic principles of CNS immunology. In: Winn HR (ed) Youman's Neurological Surgery, 5th edn. WB Saunders, Philadelphia, 2003 (in press)
Burger PC: Classification, grading, and patterns of spread of malignant gliomas. In: Apuzzo MLJ (ed) Malignant Cerebral Glioma, Park Ridge, IL: American Association of Neurological Surgeons, 1990, pp 3–17
Daumas-Duport C, Scheithauer BW, Kelly PJ: A histologic and cytologic method for the spatial definition of gliomas. Mayo Clin Proc 62: 435–449, 1987
Loeffler JS, Alexander III E, Hochberg FH, et al.: Clinical patterns of failure following stereotactic interstitial irradiation for malignant gliomas. Int J Radiat Oncol Biol Phys 19: 1455–1462, 1990
Grabowska A, Lampson LA: MHC expression in nonlymphoid tissues of the developing embryo: strongest class I or class II expression in separate populations of potential antigen-presenting cells in the skin, lung, gut, and interorgan connective tissue. Dev Comp Immunol 19: 425–450, 1995
Moller P, Hammerling GJ: The role of surface HLAA, B,C molecules in tumour immunity Cancer Surveys 13: 101–127, 1992
Lampson LA: Immunobiology of brain tumors: antigens, effectors, and delivery to sites of microscopic tumor in the brain. In: PM Black, JS Loeffler (eds) Cancer of the Nervous System. Blackwell, Boston, 1997, pp 874–906 43
Lampson LA, Hickey WF: Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from ‘histologically normal’ to that showing different levels of glial tumor involvement. J Immunol 136: 4054–4062, 1986
Lampson LA: Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Micros Res Tech 32: 267–285, 1995
Phillips LM, Simon P, Lampson LA: Site-specific immune regulation in the brain: differential modulation of MHC proteins in brainstem vs. hippocampus. J Comp Neurol 405: 322–333, 1999
McVicar DW, Davis DF, Merchant RE: In vitro analysis of the proliferative potential of T cells from patients with brain tumor: glioma-associated immunosuppression unrelated to intrinsic cellular defect. J Neurosurg 76: 251–260, 1992
Zou JP, Morford LA, Chougnet C, et al.: Human gliomainduced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol 162: 4882–4892, 1999
Spence AM, Priestly G: A survey of ethylnitrosoureainduced rat gliomas for the presence of tumour rejection antigens expressed in vivo. Neuropath Appl Neurobiol 7: 63–75, 1981
Benda P, Lightbody J, Sato G, Levine L, Sweet W: Differentiated rat glial cell strain in tissue culture. Science 161: 370–371, 1968
Barth RF: Rat brain tumor models in experimental neurooncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neuro-Oncol 36: 91–102, 1998
Schmidek HH, Nielsen SL, Schiller AL, Messer J: Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg 34: 335–340, 1971
Albright L, Seab JA, Ommaya AK: Intracerebral delayed hypersensitivity reactions in glioblastoma multiforme patients. Cancer 39: 1331–1336, 1977
Geyer D, Landay A: Immunogenetic and immunologic aspects of gliosarcoma growth in rats. Lab Invest 49: 436–444, 1983
Lampson LA, Chen A, Vortmeyer AO, Sloan AE, Ghogawala Z, Kim L: Enhanced T cell migration to sites of microscopic CNS disease: complementary treatments evaluated by 2-and 3-D image analysis. Brain Pathol 4: 125–134, 1994
Lampson LA, Lampson MA, Dunne AD: Exploiting the lacZ reporter gene for quantitative analysis of disseminated tumor growth within the brain: use of the lacZ gene product as a tumor antigen, for evaluation of antigenic modulation, and to facilitate image analysis of tumor growth in situ. Cancer Res 53: 176–182, 1993
Lampson LA, Wen P, Roman VA, Morris JH, Sarid J: Disseminating tumor cells and their interactions with leukocytes visualized in the brain. Cancer Res 52: 1018–1025, 1992
Sethna M, Lampson LA: Immune modulation within the brain: recruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of IFN-γ. J Neuroimmunol 34: 121–132, 1991
Paxinos G, Watson G: The Rat Brain in Stereotaxic Coordinates, 2nd edn. Academic Press, New York, 1986
Cserr HF, Ostrach LH: Bulk flow of interstitial fluid after intracranial injection of Blue Dextran 2000. Exp Neurol 45: 50–60, 1974
Zhang ET, Richards HK, Kida S, Weller RO: Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropath 83: 233–239, 1992
Peterson DL, Brown Jr WE: Animal models for brain tumors. In: PM Black, Loeffler JS (eds) Cancer of the Nervous System. Boston, Blackwell, 1997, pp 829–842
Lampson LA, Lampson MA, Dunne A, Sethna MP: Tumor/cytokine interactions within the brain: interactions between gamma interferon and visualizable brain tumor cells. Neurol 41(Suppl 1): 302, 1991
Wen P, Lampson MA, Lampson LA: Effects of gamma interferon on major histocompatibility complex expression and lymphocytic infiltration in the 9L gliosarcoma brain tumor model: implications for strategies of immunotherapy. J Neuroimmunol 36: 57–68, 1992
Oxenius A, Bachmann MF: Similar ligand densities required for restimulation and effector function of cytotoxic T cell. Cell Immunol 179: 16–21, 1997
Croft, M: Activation of naive, memory and effector T cells. Curr Opin Immunol 6: 431–437, 1994
Dhib-Jalbut S, Gogate N, Jiang H, Eisenberg H, Bergey G: Human microglia activate lymphoproliferative responses to recall viral antigens. J Neuroimmunol 65: 67–73, 1996
Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A: Antigen presentation and tumor cytotoxicity by interferon-? treated microglial cells. Eur J Immunol 17: 1271–1278, 1987
Hickey WF, Kimura, H: Perivascular microglial cells of the CNS are bone marrow derived and present antigen in vivo. Science 239: 290–292, 1988
Myers KJ, Dougherty JP, Ron Y: In vivo antigen presentation by both brain parenchymal cells and hematopoietically derived cells during the induction of experimental autoimmune encephalomyelitis. J Immunol 151: 2252–2260, 1993
Sedgwick JD, Ford AL, Foulcher E, Airriess R: Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol 160: 5320–5330, 1998
Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG: Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer 58: 240–247, 1994
Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST: Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol (Berl) 93: 518–527, 1997
Gehrmann J, Matsumoto Y, Kreutzberg GW: Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev 20: 269–287, 1995
Folkman J, Klagsbrun M: Angiogenic factors. Science 235: 442–447, 1987
Greenberg P: Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol 49: 281–355, 1991
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H: The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188: 2357–2368, 1998
Roggendorf, W, Strupp S, Paulus W: Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol (Berl) 92: 288–293, 1996
Lorusso L, Rossi ML: The phagocyte in human gliomas. Ann NY Acad Sci 832: 405–425, 1997
Fries G, Perneczky A, Kempski O: Glioblastoma-associated circulating monocytes and the release of epidermal growth factor. J Neurosurg 85: 642–647, 1996
Killion JJ, Fidler IJ: Therapy of cancer metastasis by tumoricidal activation of tissue macrophages using lipsomeencapsulated immunomodulators. Pharmacol Ther 78: 141–154, 1998
Kirsch M, Fischer H, Schackert G: Activated monocytes kill malignant brain tumor cells in vitro. J Neuro-Oncol 20: 35–45, 1994
Lampson LA, Kushner PD, Sobel RA: Major histocompatibility antigen expression in the affected tissues in amyotrophic lateral sclerosis. Ann Neurol 28: 365–372, 1990
Lampson LA: Brain tumor immunotherapy: an immunologist's view. This special issue
Lampson LA. Immune regulation in the brain: lessons from autoimmunity, implications for brain tumor therapy. In: Ali-Osman F (ed) Human Brain Tumors, Humana, 2003, (in press)
Lampson LA. New animal models to probe brain tumor biology, therapy, and immunotherapy: advantages and remaining concerns. J Neuro-Oncol 53: 275–287, 2001
Kapoor R, Lampson LA: A model for blood-borne parenchymal metastasis of mammary carcinoma and effect of a needle wound. J Neuropathol Exp Neurol, 2003 (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dutta, T., Spence, A. & Lampson, L.A. Robust Ability of IFN-γ to Upregulate Class II MHC Antigen Expression in Tumor Bearing Rat Brains. J Neurooncol 64, 31–44 (2003). https://doi.org/10.1023/A:1024973506989
Issue Date:
DOI: https://doi.org/10.1023/A:1024973506989