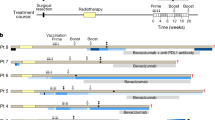
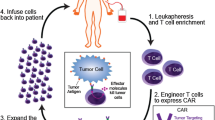
Abstract
Glioblastoma is a highly aggressive brain tumor with limited treatment options. Several major challenges have limited the development of novel therapeutics, including the extensive heterogeneity of tumor cell states within each glioblastoma and the ability of glioma cells to diffusely infiltrate into neighboring healthy brain tissue, including the contralateral hemisphere. A T cell-mediated immune response could deal with these challenges based on the ability of polyclonal T cell populations to recognize diverse tumor antigens and perform surveillance throughout tissues. Here we will discuss the major pathways that inhibit T cell-mediated immunity against glioblastoma, with an emphasis on receptor–ligand systems by which glioma cells and recruited myeloid cells inhibit T cell function. A related challenge is that glioblastomas tend to be poorly infiltrated by T cells, which is not only caused by inhibitory molecular pathways but also currently utilized drugs, in particular high-dose corticosteroids that kill activated, proliferating T cells. We will discuss innovative approaches to induce glioblastoma-directed T cell responses, including neoantigen-based vaccines and sophisticated CAR T cell approaches that can target heterogeneous glioblastoma cell populations. Finally, we will propose a conceptual framework for the future development of T cell-based immunotherapies for glioblastoma.



Similar content being viewed by others
References
Neftel C et al (2019) An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178(4):835-849 e21
Patel AP et al (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344(6190):1396–1401
Tirosh I, Suva ML (2020) Tackling the Many Facets of Glioblastoma Heterogeneity. Cell Stem Cell 26(3):303–304
Chaligne R et al (2021) Epigenetic encoding, heritability and plasticity of glioma transcriptional cell states. Nat Genet 53(10):1469–1479
Rosen J et al (2018) Extracranial Metastases of a Cerebral Glioblastoma: A Case Report and Review of the Literature. Case Rep Oncol 11(2):591–600
Jusue-Torres I, Prabhu VC, Jones GA 2021 Dandy's hemispherectomies: historical vignette. J Neurosurg, 1–7
Dandy WE (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia: preliminary report. J Am Med Assoc 90:823–825
Brown TJ et al (2016) Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol 2(11):1460–1469
Duffau H (2013) A new philosophy in surgery for diffuse low-grade glioma (DLGG): oncological and functional outcomes. Neurochirurgie 59(1):2–8
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 6(4):753–64
Sanai N et al (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115(1):3–8
Nieder C et al (2005) Treatment of unresectable glioblastoma multiforme. Anticancer Res 25(6C):4605–4610
Simpson JR et al (1993) Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 26(2):239–244
van Tellingen O et al (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 19:1–12
Friebel E et al (2020) Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell 181(7):1626–164220
Chongsathidkiet P et al (2018) Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 24(9):1459–1468
Mathewson ND et al (2021) Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell 184(5):1281-1298 e26
Iorgulescu JB et al (2021) Concurrent Dexamethasone Limits the Clinical Benefit of Immune Checkpoint Blockade in Glioblastoma. Clin Cancer Res 27(1):276–287
Sengupta S et al (2012) Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol 2012:831090
Song AJ et al (2021) Impact of lymphopenia on survival for elderly patients with glioblastoma: A secondary analysis of the CCTG CE6 (EORTC 26062–22061 TROG0301) randomized clinical trial. Neurooncol Adv 3(1):vdab153
Bai X et al (2021) Efficacy of bevacizumab in the treatment of refractory brain edema of metastatic tumors from different sources. Neurol Res 43(12):955–960
Kurkjian C, Kim ES (2012) Risks and benefits with bevacizumab: evidence and clinical implications. Ther Adv Drug Saf 3(2):59–69
Banks PD et al (2019) Bevacizumab as a steroid-sparing agent during immunotherapy for melanoma brain metastases: A case series. Health Sci Rep 2(3):e115
Reardon DA et al (2020) Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 6(7):1003–1010
Bander ED et al (2021) Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer 127(12):2062–2073
Li, W., et al 2021 Efficacy of PD-1/L1 inhibitors in brain metastases of non-small-cell lung cancer: pooled analysis from seven randomized controlled trials. Future Oncol
Sun BL et al (2018) Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog Neurobiol 163–164:118–143
Louveau A et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Song E et al (2020) VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577(7792):689–694
Engelhardt B (2006) Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm (Vienna) 113(4):477–485
Johnson LA et al (2015) Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 7(275):275ra22
O’Rourke DM et al (2017) A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9(399):eaaa0984
Wei J et al (2019) Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest 129(1):137–149
Bhat KPL et al (2013) Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24(3):331–346
Gangoso E et al (2021) Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell 184(9):2454-2470 e26
Ramkissoon LA et al (2020) Genomic Profiling of Circulating Tumor DNA From Cerebrospinal Fluid to Guide Clinical Decision Making for Patients With Primary and Metastatic Brain Tumors. Front Neurol 11:544680
Shankar GM et al (2017) Liquid biopsy for brain tumors. Expert Rev Mol Diagn 17(10):943–947
Siravegna G et al (2017) Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 14(9):531–548
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541(7637):321–330
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18(3):153–167
Tumeh PC et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571
Klempner SJ et al (2020) Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. Oncologist 25(1):e147–e159
Gromeier M et al (2021) Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun 12(1):352
Rosen DB et al (2005) Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol 175(12):7796–7799
Sun Y et al (2021) Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 184(2):404-421 e16
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 44(5):989–1004
Tawbi HA et al (2022) Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 386(1):24–34
Harris-Bookman S et al (2018) Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int J Cancer 143(12):3201–3208
Kim JE et al (2017) Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin Cancer Res 23(1):124–136
Baumeister SH et al (2016) Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 34:539–573
Hodi FS et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Larkin J et al (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373(1):23–34
Genoud V et al (2018) Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology 7(12):e1501137
Sanjabi S, Oh SA, Li MO (2017) Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol 9(6)
Munger JS et al (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96(3):319–328
Guerrero PA et al (2017) Glioblastoma stem cells exploit the alphavbeta8 integrin-TGFbeta1 signaling axis to drive tumor initiation and progression. Oncogene 36(47):6568–6580
Gabriely G, Quintana FJ (2020) Role of AHR in the control of GBM-associated myeloid cells. Semin Cancer Biol 64:13–18
Takenaka MC et al (2019) Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci 22(5):729–740
Bunse L et al (2018) Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 24(8):1192–1203
Hilf N et al (2019) Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565(7738):240–245
Keskin DB et al (2019) Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565(7738):234–239
Platten M et al (2021) A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 592(7854):463–468
Kreiter S et al (2015) Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520(7549):692–696
Maude SL et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517
O'Rourke D.M et al. (2017) A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9(399)
Park JH et al (2018) Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 378(5):449–459
Choi BD et al (2019) CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 37(9):1049–1058
Krebs S et al (2014) T cells redirected to interleukin-13Ralpha2 with interleukin-13 mutein–chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Ralpha1. Cytotherapy 16(8):1121–1131
Lin Q et al (2021) First-in-Human Trial of EphA2-Redirected CAR T-Cells in Patients With Recurrent Glioblastoma: A Preliminary Report of Three Cases at the Starting Dose. Front Oncol 11:694941
Mount CW et al (2018) Potent antitumor efficacy of anti-GD2 CAR T cells in H3–K27M(+) diffuse midline gliomas. Nat Med 24(5):572–579
Morsut L et al (2016) Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 164(4):780–791
Choe JH, et al. (2021) SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med 13(591).
Menzies AM et al (2021) Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 27(2):301–309
Acknowledgements
This work was supported by a grant from NIH (P01 CA236749 to K.W.W.) and The Jennifer Oppenheimer Cancer Research Initiative at Dana-Farber Cancer Institute (to K.W.W.). S.M. is supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG, grant MA 8489/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
K.W.W. serves on the scientific advisory board of T-Scan Therapeutics, SQZ Biotech and Nextechinvest and receives sponsored research funding from Novartis. He is a scientific co-founder of Immunitas Therapeutics.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on: Neuroimmune Interactions in Health and Disease - Guest Editors: David Hafler & Lauren Sansing
Rights and permissions
About this article
Cite this article
Marx, S., Godicelj, A. & Wucherpfennig, K.W. A Conceptual Framework for Inducing T Cell-Mediated Immunity Against Glioblastoma. Semin Immunopathol 44, 697–707 (2022). https://doi.org/10.1007/s00281-022-00945-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-022-00945-5