Abstract
Once cancer cells have spread and formed secondary masses, breast cancers are largely incurable even with state-of-the-art medicine. To improve diagnosis and therapy, better markers are needed to distinguish cells which have a high probability for causing clinically relevant, macroscopic metastases. In this review, we summarize the several genes that regulate breast cancer metastasis. Two categories of genes are presented—metastasis activator (ras, MEK1, mta1, proteinases, adhesion molecules, chemoattractants/receptors, autotaxin, PKC, S100A4, RhoC, osteopontin) and metastasis suppressor (Nm23, E-cadherin, TIMPs, KiSS1, Kai1, Maspin, MKK4, BRMS1). While the mechanisms of action for most of these genes are not fully elucidated, some clues are emerging and are presented.
Similar content being viewed by others
REFERENCES
D. R. Welch and L. L. Wei (1998). Genetic and epigenetic regulation of human breast cancer progression and metastasis. Endocr. Relat. Cancer 5:155-197.
B. A. Yoshida, M. Sokoloff, D. R. Welch, and C. W. Rinker-Schaeffer (2000). Metastasis-suppressor genes: A review and perspective on an emerging field. J. Natl. Cancer Inst. 92:1717-1730.
D. R. Welch and C. W. Rinker-Schaeffer (1999). What defines a useful marker of metastasis in human cancer? J. Natl. Cancer Inst. 91:1351-1353.
I. J. Fidler and R. Radinsky (1990). Genetic control of cancer metastasis. J. Natl. Cancer Inst. 82:166-168.
S. F. Goldberg, J. F. Harms, K. Quon, and D. R. Welch (1999). Metastasis-suppressed C8161 melanoma cells arrest in lung but fail to proliferate. Clin. Exp. Metastasis 17: 601-607.
M. A. Chekmareva, M. M. Kadkhodaian, C. M. P. Hollowell, H. Kim, B. A. Yoshida, H. H. Luu, W. M. Stadler, and C.W. Rinker-Schaeffer (1998). Chromosome 17-mediated dormancy of AT6.1 prostate cancer micrometastases. Cancer Res. 58:4963-4969.
D. R. Welch (1997). Technical considerations for studying cancer metastasis in vivo. Clin. Exp. Metastasis 15:272-306.
J. E. Price, A. Polyzos, R. D. Zhang, and L. M. Daniels (1990). Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 50:717-721.
J. E. Price and R.D. Zhang (1990). Studies of human breast cancer metastasis using nude mice. Cancer Metastasis Rev. 8:285-297.
S. M. Frisch and E. Ruoslahti (1997). Integrins and anoikis. Curr. Opin. Cell Biol. 9:701-706.
B. Mann, A. Gratchev, C. Bohm, M. L. Hanski, H. D. Foss, G. Demel, B. Trojanek, I. Schmidt-Wolf, H. Stein, E. O. Riecken, H. J. Buhr, and C. Hanski (1999). FasL is more frequently expressed in liver metastases of colorectal cancer than in matched primary carcinomas. Br. J. Cancer 79:1262-1269.
T. A. Springer (1994). Traffic signals for lymphocyte recirculation and leukocyte emigration: A multistep paradigm. Cell 76:301-314.
T. Krause and G. A. Turner (1999). Are selectins involved in metastasis? Clin. Exp. Metastasis 17:183-192.
C. C. Kumar (1998). Signaling by integrin receptors. Oncogene 17:1365-1373.
A. Raz and R. Lotan (1987). Endogenous galactoside-binding lectins: A new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 6:433-452.
A. K. Perl, P. Wilgenbus, U. Dahl, H. Semb, and G. Christofori (1998). A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature (London) 392:190-193.
T. Akimoto, S. Kawabe, A. Grothey, and L. Milas (1999). Low E-cadherin and beta-catenin expression correlates with increased spontaneous and artificial lung metastases of murine carcinomas. Clin. Exp. Metastasis 17:171-176.
K. Brew, D. Dinakarpandian, and H. Nagase (2000). Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1477:267-283.
A. R. Nelson, B. Fingleton, M. L. Rothenberg, and L. M. Matrisian (2000). Matrix metalloproteinases: Biologic activity and clinical implications. J. Clin. Oncol. 18:1135-1149.
A. F. Chambers and L. M. Matrisian (1997). Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 89:1260-1270.
P. A. Andreasen, L. Kjoller, L. Christensen, and M. J. Duffy (1997). The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer 72:1-22.
J. E. Koblinski, M. Ahram, and B. F. Sloane (2000). Unraveling the role of proteases in cancer. Clin. Chim. Acta 291:113-135.
M. L. Stracke, T. Clair, and L. A. Liotta (1997). Autotaxin, tumor motility-stimulating exophosphodiesterase. Adv. Enzyme Regul. 37:135-144.
K. Lamszus, L. Jin, A. Fuchs, E. Shi, S. Chowdhury, Y. Yao, P. J. Polverini, J. Laterra, I. D. Goldberg, and E. M. Rosen (1997). Scatter factor stimulates tumor growth and tumor angiogenesis in human breast cancers in the mammary fat pads of nude mice. Lab. Invest. 76:339-353.
K. Jacob, M. Webber, D. Benayahu, and H. K. Kleinman (1999). Osteonectin promotes prostate cancer cell migration and invasion: Apossible mechanism for metastasis to bone. Cancer Res. 59:4453-4457.
A. Müller, B. Homey, H. Soto, N. F. Ge, D. Catron, M. E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S. N. Wagner, J. L. Barrera, A. Mohar, E. Verástegui, and A. Zlotnik (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature (London) 410:50-56.
Y. Toh, S. D. Pencil, and G. L. Nicolson (1994). A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J. Biol. Chem. 269:22958-22963.
A. Neri, D. R. Welch, T. Kawaguchi, and G. L. Nicolson (1982). Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J. Natl. Cancer Inst. 68:507-517.
M. D. Martin, K. Fischbach, C. K. Osborne, S. K. Mohsin, D. C. Allred, and P. O'Connell (2001). Loss of heterozygosity events impeding breast cancer metastasis contain the MTA1 gene. Cancer Res. 61:3578-3580.
Y. Xue, J. Wong, G. T. Moreno, M. K. Young, J. Cote, and W. Wang (1998). NURD, a novel complex with both ATPdependent chromatin-remodeling and histone deacetylase activities. Mol. Cell 2:851-861.
Y. Zhang, H. H. Ng, H. Erdjument-Bromage, P. Tempst, A. Bird, and D. Reinberg (1999). Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 13:1924-1935.
M. Schwirzke, S. Schiemann, A. U. Gnirke, and U. H. Weidle (1999). New genes potentially involved in breast cancer metastasis. Anticancer Res. 19:1801-1814.
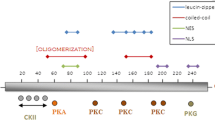
S. C. Kiley, K. J. Clark, S. K. Duddy, D. R. Welch, and S. Jaken (1999). Increased protein kinase C± in mammary tumor cells: Relationship to transformation and metastatic progression. Oncogene 18:6748-6757.
S. C. Kiley, K. J. Clark, M. Goodnough, D. R. Welch, and S. Jaken (1999). Protein kinase C delta involvement in mammary tumor cell metastasis. Cancer Res. 59:3230-3238.
A. F. Chambers and A. B. Tuck (1993). Ras-responsive genes and tumor metastasis. Crit. Rev. Oncog. 4:95-114.
C. P. Webb, G. A. Taylor, M. Jeffers, M. Fiscella, M. Oskarsson, J. H. Resau, and G. F. Vande Woude (1998). Evidence for a role of Met-HGF/SF during Ras-mediated tumorigenesis/ metastasis. Oncogene 17:2019-2025.
D. R. Welch, T. Sakamaki, R. Pioquinto, T. O. Leonard, S. F. Goldberg, Q. Hon, M. Rieber, M. Strasberg-Rieber, D. J. Hicks, J. V. Bonventre, and A. Alessandrini (2000). Transfection of constitutively active Mek1 confers tumorigenic and metastatic potentials to NIH3T3 cells. Cancer Res. 60:1552-1556.
A. A. P. Schmitz, E. E. Govek, B. Böttner, and L. Van Aelst (2000). Rho GTPases: Signaling, migration, and invasion. Exp. Cell Res. 261:1-12.
E. E. Sander and J.G. Collard (1999). Rho-like GTPases: Their role in epithelial cell-cell adhesion and invasion. Eur. J. Cancer [A] 35:1302-1308.
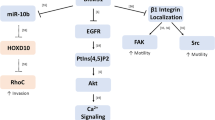
K. L. Van Golen, Z. F. Wu, X. T. Qiao, L. W. Bao, and S. D. Merajver (2000). RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 60:5832-5838.
A. J. Oates, R. Barraclough, and P. S. Rudland (1997). The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis 17:1-15.
H. Singhal, D. S. Bautista, K. S. Tonkin, F. P. O'Malley, A. B. Tuck, A. F. Chambers, and J. F. Harris (1997). Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin. Cancer Res. 3:605-611.
A. B. Tuck, F. P. O'Malley, H. Singhal, J. F. Harris, K. S. Tonkin, N. Kerkvliet, Z. Saad, G. S. Doig, and A. F. Chambers (1998). Osteopontin expression in a group of lymph node negative breast cancer patients. Int. J. Cancer 79:502-508.
Y.W. Kim, Y. K. Park, J. Lee, S.W. Ko, and M. H. Yang (1998). Expression of osteopontin and osteonectin in breast cancer. J. Korean Med. Sci. 13:652-657.
C. Gruss and M. Herlyn (2001). Role of cadherins and matrixins in melanoma. Curr. Opin. Oncol. 13:117-123.
T. A. Graham, C. Weaver, F. Mao, D. Kimelman, and W. Q. Xu (2000). Crystal structure of a β-catenin/Tcf complex. Cell 103:885-896.
G. Berx, A. M. Cleton-Jansen, K. Strumane, W. J. F. De Leeuw, F. Nollet, F. Van Roy, and C. Cornelisse (1996). E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 13:1919-1925.
J. R. Graff, E. Gabrielson, H. Fujii, S. B. Baylin, and J. G. Herman (2000). Methylation patterns of the E-cadherin 5'CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J. Biol. Chem. 275:2727-2732.
K. M. Hajra, X. Ji, and E. R. Fearon (1999). Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene 18:7274-7279.
G. Christofori and H. Semb (1999). The role of the celladhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci. 24:73-76.
M. Toi, S. Ishigaki, and T. Tominaga (1998). Metalloproteinases and tissue inhibitors of metalloproteinases. Breast Cancer Res. Treat. 52:113-124.
A. H. Ree, V. A. Florenes, J. P. Berg, G. M. Maelandsmo, J. M. Nesland, and O. Fodstad (1997). High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin. Cancer Res. 3:1623-1628.
G. Y. Li, R. Fridman, and H. R. C. Kim (1999). Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 59:6267-6275.
K. McCarthy, T. Maguire, G. McGreal, E. McDermott, N. O'Higgins, and M. J. Duffy (1999). High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int. J. Cancer 84:44-48.
J. M. Kozlowski, I. J. Fidler, D. Campbell, Z. Xu, M. E. Kaighn, and I. R. Hart (1984). Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 44:3522-3529.
W. H. Yu, S. S. C. Yu, Q. Meng, K. Brew, and J. F. Woessner Jr. (2000). TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J. Biol. Chem. 275:31226-31232.
J. M. Freije, N. J. MacDonald, and P. S. Steeg (1998). Nm23 and tumour metastasis: Basic and translational advances. Biochem. Soc. Symp. 63:261-271.
D. Lombardi, M. L. Lacombe, and M. G. Paggi (2000). nm23: Unraveling its biological function in cell differentiation. J. Cell Physiol. 182:144-149.
M. T. Hartsough, S. E. Clare, M. Mair, A. G. Elkahloun, D. Sgroi, C. K. Osborne, G. Clark, and P. S. Steeg (2001). Elevation of breast carcinoma Nm23-H1 metastasis suppressor gene expression and reduced motility by DNAmethylation inhibition. Cancer Res. 61:2320-2327.
E. H. Postel, S. J. Berberich, S. J. Flint, and C. A. Ferrone (1993). Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science (Washington, D.C.) 261:478-480.
N. J. MacDonald, A. De La Rosa, M. A. Benedict, J. M. Freije, H. Krutsch, and P. S. Steeg (1993). A serine phosphorylation of Nm23, and not its nucleoside diphosphate kinase activity, correlates with suppression of tumor metastatic potential. J. Biol. Chem. 268:25780-25789.
A. L. Perraud, V. Weiss, and R. Gross (1999). Signalling pathways in two-component phosphorelay systems. Trends Microbiol. 7:115-120.
A. R. Shenoy (2000). His kinase or mine? Histidine kinases through evolution. J. Biosci. 25:317-322.
S. I. Aizawa, C. S. Harwood, and R. J. Kadner (2000). Signaling components in bacterial locomotion and sensory reception. J. Bacteriol. 182:1459-1471.
R. Sager, S. Sheng, P. Pemberton, and M. J. Hendrix (1996). Maspin: A tumor suppressing serpin. Curr. Top. Microbiol. Immunol. 213:51-64.
F. E. Domann, J. C. Rice, M. J. C. Hendrix, and B.W. Futscher (2000). Epigenetic silencing of maspin gene expression in human breast cancers. Int. J. Cancer 85:805-810.
S. Zucker, J. Cao, and W.T. Chen (2000). Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19:6642-6650.
J. R. Starkey, H. L. Hosick, D. R. Stanford, and H. D. Liggitt (1984). Interaction of metastatic tumor cells with bovine lens capsule basement membrane. Cancer Res. 44:1585-1594.
E. Friedman, C. Urmacher, and S. Winawer (1984). A model for human colon carcinoma evolution based on the differential response of cultured preneoplastic, premalignant and malignant cells to 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 44:1568-1578.
M. Zhang, O. Volpert, Y. H. Shi, and N. Bouck (2000). Maspin is an angiogenesis inhibitor. Nat. Med. 6:196-199.
J. LeCouter, J. Kowalski, J. Foster, P. Hass, Z. M. Zhang, L. Dillard-Telm, G. Frantz, L. Rangell, L. DeGuzman, G. A. Keller, F. Peale, A. Gurney, K. J. Hillan, and N. Ferrara (2001). Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412:877-884.
M. Zhang, Y. Shi, D. Magit, P. A. Furth, and R. Sager (2000). Reduced mammary tumor progression in WAP-TAg/WAPmaspin bitransgenic mice. Oncogene 19:6053-6058.
S. M. North and G. L. Nicolson (1985). Heterogeneity in the sensitivities of the 13762NF rat mammary adenocarcinoma cell clones to cytolysis mediated by extra-and intratumoral macrophages. Cancer Res. 45:1453-1458.
J. J. Li, N. H. Colburn, and L.W. Oberley (1998). Maspin gene expression in tumor suppression induced by overexpressing manganese-containing superoxide dismutase cDNA in human breast cancer cells. Carcinogenesis 19:833-839.
J.-T. Dong, P. W. Lamb, C. W. Rinker-Schaeffer, J. Vukanovic, T. Ichikawa, J. T. Isaacs, and J. C. Barrett (1995). KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science (Washington, D.C.) 268:884-886.
K. K. Phillips, A. E. White, D. J. Hicks, D. R. Welch, J. C. Barrett, L. L. Wei, and B. E. Weissman (1998). Correlation between reduction of metastasis in the MDA-MB-435 model system and increased expression of the Kai-1 protein. Mol. Carcinog. 21:111-120.
X. H. Yang, D. R. Welch, K. K. Phillips, B. E. Weissman, and L. L. Wei (1997). KAI1, a putative marker for metastatic potential in human breast cancer. Cancer Lett. 119:149-155.
C. I. Huang, N. Kohno, E. Ogawa, M. Adachi, T. Taki, and M. Miyake (1998). Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am. J. Pathol. 153:973-983.
X. Yang, L. Wei, C. Tang, R. Slack, E. Montgomery, and M. Lippman (2000). KAI1 protein is down-regulated during the progression of human breast cancer. Clin. Cancer Res. 6:3424-3429.
P. Jackson, D. Millar, E. Kingsley, G. Yardley, K. Ow, S. Clark, and P. J. Russell (2000). Methylation of a CpG island within the promoter region of the KAI1 metastasis suppressor gene is not responsible for down-regulation of KAI1 expression in invasive cancers or cancer cell lines. Cancer Lett. 157:169-176.
T. Mashimo, M. Watabe, S. Hirota, S. Hosobe, K. Miura, P. J. Tegtmeyer, C.W. Rinker-Schaeffer, and K. Watabe (1998). The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc. Natl. Acad. Sci.U.S.A. 95:11307-11311.
C. Duriez, N. Falette, U. Cortes, C. Moyret-Lalle, and A. Puisieux (2000). Absence of p53-dependent induction of the metastatic suppressor KAI1 gene after DNA damage. Oncogene 19:2461-2464.
A. West, P. J. Vojta, D. R. Welch, and B. E. Weissman (1998). Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics 54:145-148.
J.-H. Lee and D. R. Welch (1997). Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 57:2384-2387.
A. I. Muir, L. Chamberlain, N. A. Elshourbagy, D. Michalovich, D. J. Moore, A. Calamari, P. G. Szekeres, H. M. Sarau, J. K. Chambers, P. Murdock, K. Steplewski, U. Shabon, J. E. Miller, S. E. Middleton, J.G. Darker, C.G. C. Larminie, S. Wilson, D. J. Bergsma, P. Emson, R. Faull, K. L. Philpott, and D. C. Harrison (2001). AXOR12: A novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 276:28969-28975.
T. Ohtaki, Y. Shintani, S. Honda, H. Matsumoto, A. Hori, K. Kanehashi, Y. Torao, S. Kumano, Y. Takatsu, Y. Matsuda, Y. Ishibashi, T. Watanabe, M. Asada, T. Yamada, M. Suenaga, C. Kitada, S. Usuki, T. Kurokawa, H. Onda, O. Nishimura, and M. Fujino (2001). Metastasis suppressor gene KiSS1 encodes peptide ligand of a G-protein-coupled receptor. Nature (London) 411:613-617.
C. H. Yan, H. Wang, and D. D. Boyd (2001). KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappaB binding to the promoter as a consequence of IB α-induced block of p65/p50 nuclear translocation. J. Biol. Chem. 276:1164-1172.
C. W. Rinker-Schaeffer, A. L. Hawkins, N. Ru, J. Dong, G. Stoica, C. A. Griffin, T. Ichikawa, J. C. Barrett, and J. T. Isaacs (1994). Differential suppression of mammary and prostate cancer metastasis by human chromosomes 17 and 11. Cancer Res. 54:6249-6256.
B. A. Yoshida, Z. Dubauskas, M. A. Chekmareva, T. R. Christiano, W. M. Stadler, and C. W. Rinker-Schaeffer (1999). Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res. 59:5483-5487.
B. A. Yoshida, Z. Dubauskas, M. A. Chekmareva, M. M. Zaucha, T. R. Christiano, A. P. Christiano, W. M. Stadler, and C.W. Rinker-Schaeffer (1999). Identification and characterization of candidate prostate cancer metastasis-suppressor genes encoded on human chromosome 17. Cancer Res. 59:5483-5487.
H. L. Kim, D. J. Van der Griend, X. Yang, D. A. Benson, Z. Dubauskas, B. A. Yoshida, M. A. Chekmareva, Y. Ichikawa, M. H. Sokoloff, P. Zhan, T. Karrison, A. Lin, W. M. Stadler, T. Ichikawa, M. A. Rubin, and C. W. Rinker-Schaeffer (2001). Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 61:2833-2837.
M. J. Seraj, R. S. Samant, M. F. Verderame, and D. R. Welch (2000). Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res. 60:2764-2769.
D. R. Welch, M.J. Seraj, R. S. Samant, T.O. Leonard, J.F. Harms, and M. F. Verderame (1999). BrMS1-A human breast cancer metastasis-suppressor gene encoded on chromosome 11q13.1-q13.2. Clin. Cancer Res. 5:66.
M. M. Saunders, M. J. Seraj, Z. Y. Li, Z. Y. Zhou, C. R. Winter, D. R. Welch, and H. J. Donahue (2001). Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res. 61:1765-1767.
F. Shirasaki, M. Takata, N. Hatta, and K. Takehara (2001). Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Research 61:7422-7425.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Debies, M.T., Welch, D.R. Genetic Basis of Human Breast Cancer Metastasis. J Mammary Gland Biol Neoplasia 6, 441–451 (2001). https://doi.org/10.1023/A:1014739131690
Issue Date:
DOI: https://doi.org/10.1023/A:1014739131690