Abstract
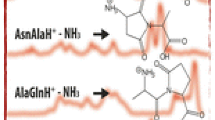
The fragmentation reactions of the singly-protonated oligoalanines trialanine to hexaalanine have been studied using energy-resolved mass spectrometry in MS2 and MS3 experiments. The primary fragmentation reactions are rationalized in terms of the bx-yz pathway of amide bond cleavage which results in formation of a proton-bound complex of an oxazolone and a peptide/amino acid; on decomposition of this complex the species of higher proton affinity preferentially retains the proton. For protonated pentaalanine and protonated hexaalanine the major primary fragmentation reaction involves cleavage of the C-terminal amide bond to form the appropriate b ion. The lower mass b ions originate largely, if not completely, by further fragmentation of the initially formed b ion. MS3 energy-resolved experiments clearly show the fragmentation sequence bn → bn−1 → bn−2. A more minor pathway for the alanines involves the sequence bn → an → bn−1 → bn−2. The a5 ion formed from hexaalanine loses, in part, NH3 to begin the sequence of fragmentation reactions a5 → a*5 → a*4 → a*3 where a*n = an−NH3. The a*3 ion also is formed from the b3 ion by the sequence b3 → a3 → a*3 with the final step being sufficiently facile that the a3 ion is not observed with significant intensity in CID mass spectra. A cyclic structure is proposed for the a*3 ion.
Article PDF
Similar content being viewed by others
References
Whitehouse, C. M.; Dreyer, R. N.; Yamashita, M.; Fenn, J. B. Electrospray Interface for Liquid Chromatographs and Mass Spectrometers. Anal. Chem. 1985, 57, 675.
Electrospray Ionization Mass Spectrometry. Fundamentals, Instrumentation, and Applications; Cole, R. B., Ed.; Wiley: New York, 1997.
Applied Electrospray Mass Spectrometry; Pramanik, B. N.; Ganguly, A. K.; Gross, M. L., Eds.; Marcel Dekker: New York, 2002.
Karas, M.; Hillenkamp, F. Laser Desorption Ionization of Proteins with Molecular Masses Exceeding 1000 Daltons. Anal. Chem. 1988, 60, 2299.
Beavis, R. C.; Chait, B. T. Matrix-Assisted Laser Desorption-Ionization Mass Spectrometry of Proteins. Methods Enzymol. 1996, 270, 519.
Tandem Mass Spectrometry; McLafferty, F. W. Ed.; Wiley: New York, 1983.
Busch, K. L.; Glish, G. L.; McLuckey, S. A. Mass Spectrometry/Mass Spectrometry: Techniques and Applications of Tandem Mass Spectrometry; VCH: New York, 1988.
Roepstorff, P.; Fohlman, J. Proposal for a Common Nomenclature for Sequence Ions in Mass Spectra of Peptides. Biomed. Mass Spectrom. 1984, 11, 601.
Biemann, K. Contributions of Mass Spectrometry to Peptide and Protein Structure. Biomed. Env. Mass Spectrom. 1988, 16, 99.
Biemann, K. Sequencing of Peptides by Tandem Mass Spectrometry and High-Energy Collision-Induced Dissociation. Methods Enzymol. 1990, 193, 455.
Papayannopoulos, I. A. The Interpretation of Collision-Induced Dissociation Tandem Mass Spectra of Peptides. Mass Spectrom. Rev. 1995, 14, 49.
Mueller, D. R.; Eckersley, M.; Richter, W. Hydrogen Transfer Reactions in the Formation of “Y+2” Sequence Ions from Protonated Peptides. Org. Mass Spectrom. 1988, 23, 217.
Cordero, M. M.; Houser, J. J.; Wesdemiotis, C. The Neutral Products Formed During Backbone Fragmentation of Protonated Peptides in Tandem Mass Spectrometry. Anal. Chem. 1993, 65, 1594.
Yalcin, T.; Khouw, C.; Csizmadia, I. G.; Peterson, M. R.; Harrison, A. G. Why are B Ions Stable Species in Peptide Mass Spectra?. J. Am. Soc. Mass Spectrom. 1995, 6, 1165.
Yalcin, T.; Csizmadia, I. G.; Peterson, M. R.; Harrison, A. G. The Structures and Fragmentation of Bn (n≥3) Ions in Peptide Mass Spectra. J. Am. Soc. Mass Spectrom. 1996, 7, 293.
Nold, M. J.; Wesdemiotis, C.; Yalcin, T.; Harrison, A. G. Amide Bond Dissociation in Protonated Peptides. Structures of the N-Terminal Ionic and Neutral Fragments. Int. J. Mass Spectrom. Ion Processes. 1997, 164, 137.
Paizs, B.; Lendvay, G.; Vékey, K.; Suhai, S. Formation of b +2 Ions from Protonated Peptides. An ab Initio Study. Rapid Commun. Mass Spectrom. 1999, 13, 525.
Harrison, A. G.; Csizmadia, I. G.; Tang, T.-H. Structures and Fragmentation of b2 Ions in Peptide Mass Spectra. J. Am. Soc. Mass Spectrom. 2000, 11, 427.
Rodriquez, C. F.; Shoeib, T.; Chu, I. K.; Siu, K. W. M.; Hopkinson, A. C. Comparison Between Protonation, Lithiation, and Argentination of 5-Oxazolones. A Study of a Key Intermediate in Gas-Phase Peptide Sequencing. J. Phys. Chem. A 2000, 104, 5355.
Farrugia, J. M.; Taverner, T.; O’Hair, R. A. J. Side-Chain Involvement in the Fragmentation Reactions of the Protonated Methyl Esters of Histidine and Its Peptides. Int. J. Mass Spectrom. 2001, 209, 99.
Farrugia, J. M.; O’Hair, R. A. J.; Reid, G. E. Do All b2 Ions Have Oxazolone Structures? Mass Spectrometry and ab Initio Studies on Protonated N-Acyl Amino Acid Methyl Ester Model Systems. Int. J. Mass Spectrom 2001, 210/211, 71.
Ambihapathy, K.; Yalcin, T.; Leung, H.-W.; Harrison, A. G. Pathways to Immonium Ions in the Fragmentation of Protonated Peptides. J. Mass Spectrom 1997, 32, 209.
Aribi, E. L. H.; Rodriquez, C. F.; Almeida, D. R. P.; Ling, Y.; Mak, W. W.-N.; Hopkinson, A. C.; Siu, K. W. M. Elucidation of Fragmentation Mechanisms of Protonated Peptide Ions and Their Products: A Case Study on Glycylglycylglycine Using Density Functional Theory and Threshold Collision-Induced Dissociation. J. Am. Chem. Soc 2003, 125, 9229.
Yeh, R. W.; Grimsley, J. M.; Bursey, M. M. Collisionally Induced Fragmentation of Protonated Oligoalanines and Oligoglycines. Biol. Mass Spectrom. 1991, 20, 443.
Schwartz, B. L.; Bursey, M. M. Some Proline Substituent Effects in the Tandem Mass Spectrum of Protonated Pentaalanine. Biol. Mass Spectrom. 1992, 21, 92.
Laskin, J.; Denisov, E.; Futrell, J. Comparative Study of Collision-induced and Surface-Induced Dissociation. 2. Fragmentation of Small Alanine-Containing Peptides in FT-ICR MS. J. Phys. Chem. B 2001, 105, 1895.
Laskin, J.; Denisov, E.; Futrell, J. Fragmentation Energetics of Small Peptides from Multiple-Collision Activation and Surface-Induced Dissociation in FT-ICR MS. Int. J. Mass Spectrom. 2002, 219, 189.
Laskin, J.; Futrell, J. Surface-Induced Dissociation of Peptides: Kinetics and Dynamics. J. Am. Soc. Mass Spectrom. 2003, 14, 1340.
Paizs, B.; Suhai, S. Towards Understanding the Tandem Mass Spectra of Protonated Oligopeptides 1: Mechanism of Amide Bond Cleavage. J. Am. Soc. Mass Spectrom. 2004, 15, 103.
Harrison, A. G. Energy-Resolved Mass Spectrometry. A Comparison of Quadrupole Cell and Cone Voltage Collision-Induced Dissociation. Rapid Commun. Mass Spectrom. 1999, 13, 1663.
van Dongen, W. D.; van Wijk, J. I. T.; Green, B. M.; Heerma, W.; Haverkamp, J. Comparison Between Collision Induced Dissociation of Electrosprayed Protonated Peptides in the Up-Front Region and in a Low-Energy Collision Cell. Rapid Commun. Mass Spectrom 1999, 13, 1712.
Harrison, A. G. Fragmentation Reactions of Alkylphenyl Ammonium Ions. J. Mass Spectrom. 1999, 34, 1253.
Makowiecki, J.; Tolonen, A.; Uusitalo, J.; Jalonen, J. Cone Voltage and Collision Cell Collision-Induced Dissociation of Triphenylethylenes of Pharmaceutical Interest. Rapid Commun. Mass Spectrom. 2001, 15, 1506.
Buré, C.; Lange, C. Comparison of Dissociation of Ions in an Electrospray Source or a Collision Cell in Tandem Mass Spectrometry. Curr. Org. Chem. 2003, 7, 1613.
McLuckey, S. A.; Cooks, R. G. Angle- and Energy-Resolved Fragmentation from Tandem Mass Spectrometry. In Tandem Mass Spectrometry; McLafferty, F. W., Ed.; Wiley: New York, 1983; p 203.
Smith, R. D.; Loo, J. A.; Barinaga, C. J.; Edmonds, C. G.; Udseth, H. R. Collisional Activation and Collision-Activated Dissociation of Large Multiply Charged Polypeptides and Proteins Produced by Electrospray Ionization. J. Am. Soc. Mass Spectrom. 1990, 1, 53.
Chen, H.; Tabei, K.; Siegel, M. M. Biopolymer Sequencing Using a Triple Quadrupole Mass Spectrometer in the ESI Nozzle-Skimmer/Precursor Ion MS/MS Mode. J. Am. Soc. Mass Spectrom. 2001, 12, 846.
Paizs, B.; Suhai, S. Combined Quantum Chemical and RRKM Modeling of the Main Fragmentation Pathways of Protonated GGG. II. Formation of b2, y1 and y2 Ions. Rapid Commun. Mass Spectrom. 2002, 16, 375.
Paizs, B.; Suhai, S. Towards Understanding Some Ion Intensity Relationships for the Tandem Mass Spectra of Protonated Peptides. Rapid Commun. Mass Spectrom. 2002, 16, 1699.
Paizs, B.; Suhai, S.; Harrison, A. G. Experimental and Theoretical Investigation of the Main Fragmentation Pathways of Protonated H-Gly-Gly-Sar-OH and H-Gly-Sar-Sar-OH. J. Am. Soc. Mass Spectrom. 2003, 14, 1454.
Paizs, B.; Suhai, S. Fragmentation Pathways of Protonated Peptides. Mass Spectrom. Rev., in press.
Fang, D.-C.; Yalcin, T.; Tang, T.-H.; Fu, X.-Y.; Harrison, A. G.; Csizmadia, I. G. Electron Distribution in Cationic Fragments Generated Mass Spectrometrically from Peptides. J. Mol. Struct. (Theochem.) 1999, 468, 135.
Tang, T.-H.; Fang, D.-C.; Harrison, A. G.; Csizmadia, I. G. A Computational Study of the Fragmentation of b3 Ions Derived from Protonated Peptides. J. Mol. Struct. (Theochem.) 2004, 675, 79.
Gassman, P. G.; Tidwell, T. T. Electron-Deficient Carbocations. Acc. Chem. Res. 1983, 16, 279.
Grutzmacher, H.-F.; Dommröse, A.-M. Kinetic Energy Release During CO Loss by Rearrangement of α-Benzoylcarbenium Ions. Org. Mass Spectrom 1983, 18, 601.
Dommröse, A.-M.; Grutzmacher, H.-F. Destabilized Carbenium Ions. Secondary and Tertiary α-Acetylbenzyl Cation and α-Benzoylbenzyl Cation. Org. Mass Spectrom 1987, 22, 437.
Wolf, R.; Grutzmacher, H.-F. Destabilized Carbenium Ions. α-Carbomethoxy-α,α-Dimethylmethyl Cation. Org. Mass Spectrom. 1989, 24, 398.
Tkaczyk, M.; Harrison, A. G. Formation of Destabilized Carbenium Ions by Charge Inversion of Negative Ions. Int. J. Mass Spectrom. Ion Processes 1991, 109, 295.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published online November 2, 2004
Rights and permissions
About this article
Cite this article
Harrison, A.G., Young, A.B. Fragmentation of protonated oligoalanines: Amide bond cleavage and beyond. J Am Soc Mass Spectrom 15, 1810–1819 (2004). https://doi.org/10.1016/j.jasms.2004.08.015
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/j.jasms.2004.08.015