Abstract
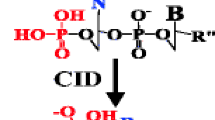
Tandem mass spectrometry is a well-established analytical tool for rapid and reliable characterization of oligonucleotides (ONs) and their gas-phase dissociation channels. The fragmentation mechanisms of native and modified nucleic acids upon different mass spectrometric activation techniques have been studied extensively, resulting in a comprehensive catalogue of backbone fragments. In this study, the fragmentation behavior of highly charged oligodeoxynucleotides (ODNs) comprising up to 15 nucleobases was investigated. It was found that ODNs exhibiting a charge level (ratio of the actual to the total possible charge) of 100% follow significantly altered dissociation pathways compared with low or medium charge levels if a terminal pyrimidine base (3' or 5') is present. The corresponding product ion spectra gave evidence for the extensive loss of a cyanate anion (NCO–), which frequently coincided with the abstraction of water from the 3'- and 5'-end in the presence of a 3'- and 5'-terminal pyrimidine nucleobase, respectively. Subsequent fragmentation of the M-NCO– ion by MS3 revealed a so far unreported consecutive excision of a metaphosphate (PO3 –)-ion for the investigated sequences. Introduction of a phosphorothioate group allowed pinpointing of PO3 – loss to the ultimate phosphate group. Several dissociation mechanisms for the release of NCO– and a metaphosphate ion were proposed and the validity of each mechanism was evaluated by the analysis of backbone- or sugar-modified ONs.

ᅟ







Similar content being viewed by others
References
Gross, J., Hillenkamp, F., Wan, K.X., Gross, M.L.: Metastable decay of negatively charged oligodeoxynucleotides analyzed with ultraviolet matrix-assisted laser desorption/ionization post-source decay and deuterium exchange. J. Am. Soc. Mass Spectrom. 12, 180–192 (2001)
McLuckey, S.A., Habibi-Goudarzi, S.: Decompositions of multiply-charged oligonucleotide anions. J. Am. Chem. Soc. 115, 12085–12095 (1993)
Wan, K.X., Gross, J., Hillenkamp, F., Gross, M.L.: Fragmentation mechanisms of oligodeoxynucleotides studied by H/D exchange and electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 12, 193–205 (2001)
Wang, Z., Wan, K.X., Ramanathan, R., Taylor, J.S., Gross, M.L.: Structure and fragmentation mechanisms of isomeric T-rich oligodeoxynucleotides: a comparison of four tandem mass spectrometric methods. J. Am. Soc. Mass Spectrom. 9, 683–691 (1998)
Schürch, S., Bernal-Mendez, E., Leumann, C.J.: Electrospray tandem mass spectrometry of mixed-sequence RNA/DNA oligonucleotides. J. Am. Soc. Mass Spectrom. 13, 936–945 (2002)
Tromp, J.M., Schürch, S.: Gas-phase dissociation of oligoribonucleotides and their analogs studied by electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 16, 1262–1268 (2005)
Andersen, T.E., Kirpekar, F., Haselmann, K.F.: RNA fragmentation in MALDI mass spectrometry studied by H/D-exchange: mechanisms of general applicability to nucleic acids. J. Am. Soc. Mass Spectrom. 17, 1353–1368 (2006)
Tromp, J.M., Schürch, S.: Electrospray ionization tandem mass spectrometry of biphenyl-modified oligo(deoxy)ribonucleotides. Rapid Commun. Mass Spectrom. 20, 2348–2354 (2006)
Keough, T., Baker, T.R., Dobson, R.L.M., Lacey, M.P., Riley, T.A., Hasselfield, J.A., Hesselberth, P.E.: Antisense DNA oligonucleotides. 2. The use of matrix-assisted laser desorption ionization mass-spectrometry for the sequence verification of methylphosphonate oligodeoxyribonucleotides. Rapid Commun. Mass Spectrom. 7, 195–200 (1993)
Bartlett, M.G., McCloskey, J.A., Manalili, S., Griffey, R.H.: The effect of backbone charge on the collision-induced dissociation of oligonucleotides. J. Mass Spectrom. 31, 1277–1283 (1996)
Wang, B.H., Hopkins, C.E., Belenky, A.B., Cohen, A.S.: Sequencing of modified oligonucleotides using in-source fragmentation and delayed pulsed ion extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Int. J. Mass Spectrom. 169, 331–350 (1997)
Monn, S.T.M., Schürch, S.: New aspects of the fragmentation mechanisms of unmodified and methylphosphonate-modified oligonucleotides. J. Am. Soc. Mass Spectrom. 18, 984–990 (2007)
Nyakas, A., Stucki, S.R., Schürch, S.: Tandem mass spectrometry of modified and platinated oligoribonucleotides. J. Am. Soc. Mass Spectrom. 22, 875–887 (2011)
Wan, K.X., Gross, M.L.: Fragmentation mechanisms of oligodeoxynucleotides: effects of replacing phosphates with methylphosphonates and thymines with other bases in T-rich sequences. J. Am. Soc. Mass Spectrom. 12, 580–589 (2001)
Nyakas, A., Eymann, M., Schürch, S.: The influence of cisplatin on the gas-phase dissociation of oligonucleotides studied by electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 20, 792–804 (2009)
Rozenski, J.: Mongo oligonucleotide mass calculator. Available at: http://mods.rna.albany.edu/masspec/Mongo-Oligo. Accessed January 2014
Rozenski, J., McCloskey, J.A.: SOS: a simple interactive program for ab initio oligonucleotide sequencing by mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 200–203 (2002)
Nyakas, A., Blum, L.C., Stucki, S.R., Reymond, J.-L., Schürch, S.: OMA and OPA software-supported mass spectra analysis of native and modified nucleic acids. J. Am. Soc. Mass Spectrom. 24, 249–256 (2013)
Yang, J., Leopold, P., Helmy, R., Parish, C., Arvary, B., Mao, B., Meng, F.: Design and application of an easy to use oligonucleotide mass calculation program. J. Am. Soc. Mass Spectrom. 24, 1315–1318 (2013)
Pan, S., Verhoeven, K., Lee, J.K.: Investigation of the initial fragmentation of oligodeoxynucleotides in a quadrupole ion trap: charge level-related base loss. J. Am. Soc. Mass Spectrom. 16, 1853–1865 (2005)
Huang, T.Y., Kharlamova, A., Liu, J., McLuckey, S.A.: Ion trap collision-induced dissociation of multiply deprotonated RNA: c/y-ions versus (a-B)/w-ions. J. Am. Soc. Mass Spectrom. 19, 1832–1840 (2008)
Rice, J.M., Dudek, G.O., Barber, M.: Mass spectra of nucleic acid derivatives—pyrimidines. J. Am. Chem. Soc. 87, 4569–4576 (1965)
Jochims, H.W., Schwell, M., Baumgartel, H., Leach, S.: Photoion mass spectrometry of adenine, thymine and uracil in the 6-22 eV photon energy range. Chem. Phys. 314, 263–282 (2005)
Flosadóttir, H.D., Jónsson, H., Sigurdsson, S.T., Ingólfsson, O.: Experimental and theoretical study of the metastable decay of negatively charged nucleosides in the gas phase. Phys. Chem. Chem. Phys. 13, 15283–15290 (2011)
Almeida, D., Antunes, R., Martins, G., Eden, S., da Silva, F.F., Nunes, Y., Garcia, G., Limao-Vieira, P.: Electron transfer-induced fragmentation of thymine and uracil in atom-molecule collisions. Phys. Chem. Chem. Phys. 13, 15657–15665 (2011)
Imhoff, M., Deng, Z.W., Huels, M.A.: Identification of ion fragments produced from thymine and deuterated thymine by low energy ion impact in films and electron impact in the gas phase. Int. J. Mass Spectrom. 245, 68–77 (2005)
Nelson, C.C., McCloskey, J.A.: Collision-induced dissociation of uracil and its derivatives. J. Am. Soc. Mass Spectrom. 5, 339–349 (1994)
Cao, H., Wang, Y.: Collisionally activated dissociation of protonated 2'-deoxycytidine, 2'-deoxyuridine, and their oxidatively damaged derivatives. J. Am. Soc. Mass Spectrom. 17, 1335–1341 (2006)
Kamel, A.M., Munson, B.: Collisionally-induced dissociation of substituted pyrimidine antiviral agents: mechanisms of ion formation using gas phase hydrogen/deuterium exchange and electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 1477–1492 (2007)
Bald, I., Flosadóttir, H.D., Ómarsson, B., Ingólfsson, O.: Metastable fragmentation of a thymidine-nucleotide and its components. Int. J. Mass Spectrom. 313, 15–20 (2012)
Arani, L.S., Mignon, P., Abdoul-Carime, H., Farizon, B., Farizon, M., Chermette, H.: DFT study of the fragmentation mechanism of uracil RNA base. Phys. Chem. Chem. Phys. 14, 9855–9870 (2012)
Guillaumont, S., Tortajada, J., Salpin, J.Y., Lamsabhi, A.M.: Experimental and computational study of the gas-phase interactions between lead(II) ions and two pyrimidic nucleobases: uracil and thymine. Int. J. Mass Spectrom. 243, 279–293 (2005)
Flosadóttir, H.D., Ómarsson, B., Bald, I., Ingólfsson, O.: Metastable decay of DNA components and their compositions—a perspective on the role of reactive electron scattering in radiation damage. Eur. Phys. J. D 66, 13 (2012)
Lamsabhi, A.M., Alcami, M., Mo, O., Yanez, M., Tortajada, J., Salpin, J.-Y.: Unimolecular reactivity of uracil-Cu2(+) complexes in the gas phase. Chem. Phys. Chem. 8, 181–187 (2007)
Beach, D.G., Gabryelski, W.: Revisiting the reactivity of uracil during collision induced dissociation: tautomerism and charge-directed processes. J. Am. Soc. Mass Spectrom. 23, 858–868 (2012)
Improta, R., Scalmani, G., Barone, V.: Radical cations of DNA bases: some insights on structure and fragmentation patterns by density functional methods. Int. J. Mass Spectrom. 201, 321–336 (2000)
da Silva, F.F., Matias, C., Almeida, D., Garcia, G., Ingólfsson, O., Flosadóttir, H.D., Ómarsson, B., Ptasinska, S., Puschnigg, B., Scheier, P., Limao-Vieira, P., Denifl, S.: NCO, a key fragment upon dissociative electron attachment and electron transfer to pyrimidine bases: site selectivity for a slow decay process. J. Am. Soc. Mass Spectrom. 24, 1787–1797 (2013)
Ziehe, M., Grossmann, T.N., Seitz, O., Linscheid, M.W.: New aspects in fragmentation of peptide nucleic acids: comparison of positive and negative ions by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 23, 1132–1138 (2009)
Bohringer, M., Roth, H.J., Hunziker, J., Gobel, M., Krishnan, R., Giger, A., Schweizer, B., Schreiber, J., Leumann, C., Eschenmoser, A.: Why pentose and not hexose nucleic-acids. 2. Preparation of oligonucleotides containing 2',3'-dideoxy-beta-d-glucopyranosyl building-blocks. Helv. Chim. Acta 75, 1416–1477 (1992)
Anusiewicz, W., Berdys-Kochanska, J., Czaplewski, C., Sobczyk, M., Daranowski, E.M., Skurski, P., Simons, J.: Charge loss in gas-phase multiply negatively charged oligonucleotides. J. Phys. Chem. A 109, 240–249 (2005)
Chandra, A.K., Nguyen, M.T., Uchimaru, T., Zeegers-Huyskens, T.: Protonation and deprotonation enthalpies of guanine and adenine and implications for the structure and energy of their complexes with water: comparison with uracil, thymine, and cytosine. J. Phys. Chem. A 103, 8853–8860 (1999)
Stucki, S.R., Desiron, C., Nyakas, A., Marti, S., Leumann, C.J., Schürch, S.: Gas-phase dissociation of homo-DNA oligonucleotides. J. Am. Soc. Mass Spectrom. 24, 1997–2006 (2013)
Acknowledgments
The authors thank C. Désiron and C. J. Leumann for providing the homoDNA sequences. Further, the authors gratefully acknowledge financial support of this work by the Swiss National Science Foundation (grant #200020_140628).
Author information
Authors and Affiliations
Corresponding author
Additional information
Adrien Nyakas and Rahel P. Eberle contributed equally to this project.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
(DOCX 1216 kb)
Supplemental Figure 2
(DOCX 498 kb)
Supplemental Figure 3
(DOCX 302 kb)
Supplemental Figure 4
(DOCX 97 kb)
Supplemental Figure 5
(DOCX 244 kb)
Supplemental Figure 6
(DOCX 523 kb)
Supplemental Figure 7
(DOCX 382 kb)
Supplemental Figure 8
(DOCX 336 kb)
Supplemental Table 1
(DOCX 53 kb)
Rights and permissions
About this article
Cite this article
Nyakas, A., Eberle, R.P., Stucki, S.R. et al. More Than Charged Base Loss — Revisiting the Fragmentation of Highly Charged Oligonucleotides. J. Am. Soc. Mass Spectrom. 25, 1155–1166 (2014). https://doi.org/10.1007/s13361-014-0873-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-014-0873-4