Abstract
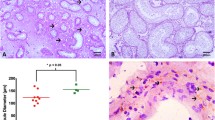
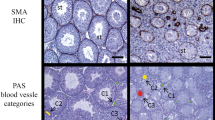
It is known that the extracellular matrix structure and composition changes with aging in many organs. Despite this, knowledge on how does the extracellular part of the ovary change with increasing age in women and how those changes might be related to women’s loss of fertility is still lacking. For this, we propose that recurrent injury and repair events on the outermost layers of the ovary due to ovulation are partly responsible for those changes women experience with aging. The histological analysis of the ovaries from 18 female-to-male transgender patients revealed that the ovarian tunica albuginea (TA) increases its thickness and density correlatively with increasing age of the patient (r = 0.52 and r = 0.55, P < 0.05 respectively). The increase in thickness is independent of the total androgen dose received and occurs because of the appearance of defined fibrotic areas underneath the TA layer which increase the total distance of dense connective tissue from the ovarian surface. In conclusion, the ovarian TA increases in its thickness and density with aging because of the appearance of fibrotic areas underneath the layer in transgender patients. This fact might contribute to reduce oocyte quality and cause ovulation difficulties in older women.





Similar content being viewed by others
Abbreviations
- GID:
-
gender identity disorder
- ECM:
-
extracellular matrix
- TA:
-
tunica albuginea
- RT:
-
room temperature
- PSR:
-
picrosirius red
References
Bongaarts J, Potter RG. 2 - natural fertility and its proximate determinants. In: Fertility, biology, and behavior. San Diego: Academic Press; 1983. p. 21–51. https://doi.org/10.1016/B978-0-08-091698-9.50007-5.
Gray RH. Biological and social interactions in the determination of late fertility. J Biosoc Sci Suppl. 1979;6:97–115.
Frank O, Bianchi PG, Campana A. The end of fertility: age, fecundity and fecundability in women. J Biosoc Sci. 1994;26(3):349–68.
Jirge PR. Poor ovarian reserve. J Hum Reprod Sci. 2016;9(2):63–9. https://doi.org/10.4103/0974-1208.183514.
Subrat P, Santa SA, Vandana J. The concepts and consequences of early ovarian ageing: a caveat to women’s health. J Reprod Infertil. 2013;14(1):3–7.
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne). 2018;9:327. https://doi.org/10.3389/fendo.2018.00327.
Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152(3):245–60. https://doi.org/10.1530/REP-16-0129.
Exbrayat J-M (2013) Histochemical and Cytochemical Methods of Visualization. Boca Raton: CRC Press. https://doi.org/10.1201/b14967.
Smith RM, Woodruff TK, Shea LD. Designing follicle-environment interactions with biomaterials. Cancer Treat Res. 2010;156:11–24. https://doi.org/10.1007/978-1-4419-6518-9_2.
Phillip JM, Aifuwa I, Walston J, Wirtz D. The mechanobiology of aging. Annu Rev Biomed Eng. 2015;17:113–41. https://doi.org/10.1146/annurev-bioeng-071114-040829.
West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–48. https://doi.org/10.1016/j.biomaterials.2007.07.001.
Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–23. https://doi.org/10.1095/biolreprod.106.054833.
Emi Y, Adachi M, Sasaki A, Nakamura Y, Nakatsuka M. Increased arterial stiffness in female-to-male transsexuals treated with androgen. J Obstet Gynaecol Res. 2008;34(5):890–7. https://doi.org/10.1111/j.1447-0756.2008.00857.x.
Elbers JM, de Jong S, Teerlink T, Asscheman H, Seidell JC, Gooren LJ. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism. 1999;48(11):1371–7.
Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132–54. https://doi.org/10.1210/jc.2009-0345.
Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, et al. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28(2):453–61. https://doi.org/10.1093/humrep/des385.
Espey LL. Ovarian proteolytic enzymes and ovulation. Biol Reprod. 1974;10(2):216–35. https://doi.org/10.1095/biolreprod10.2.216.
Hanna MD, Chizen DR, Pierson RA. Characteristics of follicular evacuation during human ovulation. Ultrasound Obstet Gynecol. 1994;4(6):488–93. https://doi.org/10.1046/j.1469-0705.1994.04060488.x.
Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front Biosci. 2003;8:s1240–8. https://doi.org/10.2741/1183.
Kular JK, Basu S, Sharma RI. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:2041731414557112. https://doi.org/10.1177/2041731414557112.
Jones MG, Andriotis OG, Roberts JJ, Lunn K, Tear VJ, Cao L, et al. Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. Elife. 2018;7. https://doi.org/10.7554/eLife.36354.
Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest. 2018;128(1):97–107. https://doi.org/10.1172/JCI93563.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. https://doi.org/10.1002/path.2277.
Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–33. https://doi.org/10.1093/humrep/dem027.
McCloskey CW, Cook DP, Kelly BS, Azzi F, Allen CH, Forsyth A, et al. Metformin abrogates age-associated ovarian fibrosis. Clin Cancer Res. 2020;26(3):632–42. https://doi.org/10.1158/1078-0432.CCR-19-0603.
Mara JN, Zhou LT, Larmore M, Johnson B, Ayiku R, Amargant F, et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging (Albany NY). 2020;12(10):9686–713. https://doi.org/10.18632/aging.103237.
Vanderhyden BC. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res. 2005;322(1):117–24. https://doi.org/10.1007/s00441-005-1100-1.
Edmonds CJ, Jasani BM, Smith T. Total body potassium and body fat estimation in relationship to height, sex, age, malnutrition and obesity. Clin Sci Mol Med. 1975;48(5):431–40. https://doi.org/10.1042/cs0480431.
Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013;1832(7):884–90. https://doi.org/10.1016/j.bbadis.2013.02.007.
Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–94. https://doi.org/10.1007/s00432-015-1974-6.
Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28(1):3–6. https://doi.org/10.1007/s10815-010-9478-4.
Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PG, et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab. 1989;69(1):151–7. https://doi.org/10.1210/jcem-69-1-151.
Pache TD, Chadha S, Gooren LJ, Hop WC, Jaarsma KW, Dommerholt HB, et al. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19(5):445–52. https://doi.org/10.1111/j.1365-2559.1991.tb00235.x.
Caanen MR, Schouten NE, Kuijper EAM, van Rijswijk J, van den Berg MH, van Dulmen-den Broeder E, et al. Effects of long-term exogenous testosterone administration on ovarian morphology, determined by transvaginal (3D) ultrasound in female-to-male transsexuals. Hum Reprod. 2017;32(7):1457–64. https://doi.org/10.1093/humrep/dex098.
Kubota T. Update in polycystic ovary syndrome: new criteria of diagnosis and treatment in Japan. Reprod Med Biol. 2013;12(3):71–7. https://doi.org/10.1007/s12522-013-0145-1.
Funding
This research was partially supported by the Sasakawa Scientific Research Grant from The Japan Science Society (Grant number: 2020-4052).
Author information
Authors and Affiliations
Contributions
PF contributed in the experimental design, performed all the histology experiments and image analysis, analyzed and interpreted all the datasets obtained, and was a major contributor in writing the manuscript.
JO contributed in the experimental design, data analysis, and the writing of the manuscript.
HF contributed in the experimental design, data analysis, and the writing of the manuscript.
MN performed the operations on all the patients, obtained and provided all the data regarding the patients’ medical history, and contributed in the writing of the manuscript.
All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information 1
A In ovarian samples stained following Masson’s Trichrome method, a moderate positive linear correlation was detected between the thickness of the TA layer and the age of the patients (r = 0.76, P < 0.001). This was congruent with the results obtained when the samples were stained following the PSR method. B From left to right, the picture shows an ovarian section from a young transgender donor (21 years old) and from an older transgender donor (44 years old) stained with Masson’s trichrome method. TA tunica albuginea, r Spearman coefficient, PSR Picrosirius red. Scale bars represent 200 μm (PNG 4361 kb).
Supplementary Information 2
Image composition depicting one representative ovarian section per each of the 18 patients participating in the study, organized by increasing age, from the lowest to the greatest. Scale bars represent 400 μm (PNG 7303 kb).
Rights and permissions
About this article
Cite this article
Ferré-Pujol, P., Otsuki, J., Funahashi, H. et al. The Thickness and Density of the Ovarian Tunica Albuginea Increases with Age in Transgender Patients. Reprod. Sci. 28, 1339–1346 (2021). https://doi.org/10.1007/s43032-020-00390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00390-5