Abstract
Giraffes exhibit a large sexual dimorphism in body size. Whether sexual dimorphisms also exist in body proportions of the axial and appendicular skeleton has been debated, particularly regarding the giraffe’s iconic long neck. We examined the anatomical proportions of the neck, forelegs, hindlegs, and body trunk of the Masai giraffe (G. tippelskirchi) in captive and wild populations. We found that female Masai giraffes have proportionally longer necks relative to their forelegs than males in contradiction to the original necks-for-sex hypothesis that proposed that the evolution of the giraffe’s long neck was driven by male-male competition. However, male neck width and apparent mass are proportionally larger than females’, supporting a modification of the necks-for-sex hypothesis. Moreover, male foreleg length is proportionally longer whereas female trunk length is proportionally longer. These sexual dimorphisms were found in both captive and wild Masai giraffes. We speculate that the initial evolution of the giraffe’s long neck and legs was driven by interspecific competition and the maternal nutritional demands of gestation and lactation through natural selection to gain a competitive advantage in browsing, and then later the neck mass was further increased as a consequence of male-male competition and sexual selection. Differences in the proportions of major body components define sex phenotypes, but several giraffes display opposite-sex phenotypes with a significantly higher level of discordancy seen in captive males. We speculate that body proportion sexual dimorphisms are maintained in the wild by natural and/or sexual selection, but in captivity selection is relaxed resulting in a higher occurrence of discordances in sexual phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body size sexual dimorphism (SSD) exists in most mammalian groups, which may be either male or female biased (Reiss 1989; Abouheif and Fairbairn 1997; Weckerly 1998; Pérez-Barbería et al. 2002). Evolutionary hypotheses for the development of SSD include natural selection based on the two sexes competing for resources or occupying different ecological niches (Slatkin 1984) or SSD may be a non-adaptive byproduct of natural selection for larger size in both sexes (Leutenegger and Cheverud 1982). For example, giraffes (Giraffa camelopardalis), a polygynous megaherbivore, exhibit extreme SSD with adult males 30–40% larger mass than adult females (Hall-Martin 1977; Mitchell 2021; Roylance-Casson 2021). The large stature difference between male and female giraffes has been suggested to be an adaptation to reduce foraging competition between the sexes (Mitchell et al. 2009; Wilkinson and Ruxton 2012; Mitchell 2021). SSD could also result from or be increased by sexual selection based on male-male competition for mates (Darwin 1871; Clutton-Brock 1989), male-female coercion (Clutton-Brock and Parker 1995), or female choice (Eberhard 1996). SSD might also be the result of some combination of the above natural and sexual selection processes. The degree of SSD differs among mammalian groups, and Darwin (1871) was the first to point out that polygyny was associated with the largest differences (Ralls 1977; Weckerly 1998; Cassini 2020). Moreover, the magnitude of SSD in mammals is typically greater in species with high body mass, known as Rensch’s rule (Rensch 1959; Abouheif and Fairbairn 1997).
Less commonly known is sexual dimorphism in body proportions (BpSD), also known as body shape dimorphisms, that are due to allometric growth and developmental differences between sexes with different overall adult sizes (Badyaev et al. 2001). Characterizing BpSD may illuminate the different selective pressures acting on the two sexes in dimorphic species (Tarnawski et al. 2014), such as when comparing the shapes of male and female skeletal components. In this study we explore body proportional sexual dimorphisms in the Masai giraffe (Giraffa tippelskirchi), which was inspired by a purported sexual dimorphism in length of the neck and an alternative theory for the evolution of the giraffe’s iconic long neck. The observation that male giraffes frequently engage in neck sparring as a means to establish dominance, led Simmons and Scheepers (1996) to propose the “necks-for-sex” theory that the giraffe’s long neck evolved via male-biased sexual selection, which proposed that longer and more muscular necks provided a mating competition advantage. A prediction of the necks-for-sex hypothesis is that males would have longer necks than females, and indeed they do (Mitchell et al. 2013). However, the longer neck in male giraffes is primarily a consequence of the overall ~ 30% larger size of males; every skeletal component is larger in adult males than adult females. Our question is: do male giraffes have proportionally longer necks than females when accounting for allometric differences between male and female skeletons? Moreover, do other aspects of the giraffe skeleton exhibit BpSD that underlie important sex-specific adaptations? The answers to these questions will identify skeletal components likely undergoing selection and inform future work using genetic pedigrees to determine the mating system of giraffes and the sex-specific body morphology traits that correlate with higher survival and/or reproduction.
Critically assessing body proportion sexual dimorphisms in giraffes requires the comparison of the appendicular and axial skeleton including the fore and hind legs and vertebral segments that contribute to the length of the neck and trunk. Given that the birthdates of wild giraffes are rarely known, previous studies by Simmons (Simmons and Scheepers 1996; Simmons and Altwegg 2010) and Mitchell (Mitchell et al. 2009; van Sittert et al. 2010; Mitchell 2021) have used body mass and size or tooth-wear as proxies for age. However, age is a confounding factor in assessing relative body proportions in giraffes because (a) their necks are known to grow at a more rapid rate than their legs during neonatal and juvenile development, (b) the body size sexual dimorphism, which is readily apparent in mature adults, develops over an unknown period of time, and (c) growth rates and terminal size are highly variable among individuals.
Our objective here was to quantify body proportions to test the existing hypotheses about BpSD of adult giraffes. Using photographic images of known-age captive Masai giraffes from North American zoos and safari parks, we calculated the relative body proportions of the neck and trunk, comprising the axial skeleton, and the forelegs and hindlegs, comprising the appendicular skeleton. Because the identity, pedigree, and birthdates of these animals were known, we were also able to quantify the postnatal developmental changes in body proportions over a wide span of ages. By utilizing photographs, we were able to obtain a larger sample size than what was possible in previous studies that utilized postmortem samples. To determine if any observed sex differences in captive giraffe body proportions also existed in wild populations, we used the same methods to estimate body proportions from photographic images of adult wild Masai giraffes located in the Tarangire Ecosystem in Tanzania.
Methods
Giraffe photographic images
We obtained photographic images of captive Masai giraffes in North American zoos and safari parks (list of institutions in supplementary data) from three sources: images taken by the authors, images obtained from zoos and safari parks, and images from online public domains including Flicker (https://www.flickr.com) and Smugmug (https://www.smugmug.com). The Association of Zoos and Aquariums (AZA) provided pedigree and vital statistical data of nearly 500 Masai giraffes that have existed in North American zoos and safari parks since the importation of the original founders from 1940 to 1982 (Cantwell 2018). To reduce inbreeding, captive giraffes have been translocated among member AZA institutions under the guidance of the AZA and the Masai giraffe species survival plan (AZA 2023). Although some of the participating AZA institutions have other giraffe subspecies, crossbreeding of Masai with other subspecies is generally not done. Moreover, the pedigree of all captive Masai giraffes in this study is known with certainty and no subspecies hybrids were included in this study. The founders of the captive Masai giraffe population in North American zoos and safari parks originated from southeastern Kenya and northeastern Tanzania (Cantwell 2018) from populations that are located east of the steep escarpments of the Gregory Rift Valley. The wild Masai giraffes used in this study (described below) are located in the same general region east of the Gregory Rift Valley escarpments and have recently been described as Eastern Masai giraffes (Lohay et al. 2023). Genetic distances measured by whole genome sequence analysis of nuclear and mitochondrial DNA sequence of several captive and wild giraffes indicates that they are closely related as compared to wild Western Masai giraffes west of Gregory Rift Valley escarpments in the Serengeti Ecosystem (DRC, unpublished results). The identities of the captive giraffes in images obtained from online photo galleries are typically not indicated. To determine the identity of giraffes in such images, we searched online for named images often associated with news announcements of newborn calves. Because the spot pattern of Masai giraffes is individually distinctive and does not change in character over their lifespan (Foster 1966), it is relatively easy to match newborn calf images with adult images. Finally, we conferred with zoo staff to verify the identities of several of the giraffes in our study. We have been unable to determine the identity of only one giraffe among the ~ 250 giraffes for which we have photographs. Images of wild adult Eastern Masai giraffes from the Tarangire Ecosystem were obtained by two of us (DEL and MLB) during triannual surveys conducted between 2011–2020 (Lee et al. 2016; Bond et al. 2021). The ages and dates of birth are unknown for the wild Masai giraffes; therefore, we used conservative estimates of height and body characteristics to identify giraffes that were highly likely to be older than 8 years of age. Specifically, males > 442 cm (14.5 ft) and females > 381 cm (12.5 ft) that exhibited mature adult body characteristics (Strauss 2014) were used in this study.
Measuring longitudinal body proportions
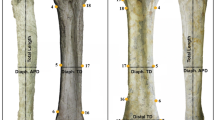
We used the GNU Image Manipulation Program (GIMP) (https://www.gimp.org/) to measure the length of the neck, trunk, foreleg, and hindleg of the body side facing the camera in units of pixels (px). These pixel measurements were used exclusively for estimating body proportions (e.g., neck length to foreleg length) within each individual and were not directly compared to other giraffes. Images, body measurement data, and body proportion calculations are deposited in ScholarSphere (see Data Accessibility). To accurately quantify body dimensions from photographic images, the body torso and neck must be perpendicular to the camera; images not perpendicular result in erroneous foreshortening of body and neck length and therefore cannot be used. The images that we utilized from the public domains as well as photos that we took have considerable variation in focal distance and lens focal length which may result in distortions in body proportion. However, such distortions are minimized by the requirement that the images must contain the full body (hooves to the top of the head) which in turn requires a long focal length, rendering the images as flat, two-dimensional images for the purpose of measuring body component lengths. In addition, the anatomical landmarks we used for length measurements need to be visible or can be accurately estimated (See Fig. 1 for measurement landmarks and definitions). Because of these stringent criteria, only a very small fraction (< 1%) of the thousands of images that we procured could be used for measurement. Body measurements (in units px) in each image were performed by one of three people, and the measurements for each image were validated by the corresponding author for accuracy.
We chose to use the neck-chest indentation, which is readily identifiable, as our primary landmark for neck and foreleg measurements. Neck length was measured from the neck indentation to the occipital ridge near the junction of the C1 cervical vertebra and the skull (Fig. 1). The estimated neck length measured between the neck indentation and occipital ridge will vary as a function of the neck angle. To provide an accurate and consistent method for estimating neck length, the neck angle must be the same for each giraffe or the neck angle must be adjusted to a standard angle. Because the resting neck angle is approximately 43°, this angle was chosen as the standard angle. Giraffes whose neck angle deviated more than 1° from 43o were adjusted by moving the occipital ridge landmark to 43° while keeping the wNeck length the same (Fig. S1). Then, the neck length was measured between the adjusted occipital ridge landmark and neck indentation (dashed blue line). The neck angle deviated more than 1° (< 42° or > 44°) in 89% of the giraffes measured and this adjustment was made. The rationale for this adjustment can be explained by considering a triangle defined by the occipital ridge (A), withers peak (B), and neck indentation (C) (Fig. S1). At rest, this triangle is nearly a right triangle whereas when a giraffe neck becomes more elevated (e.g. neck angle 63°), the triangle becomes obtuse. Given that the lengths of sides A and B remain constant, side C (Ni-OR distance) significantly lengthens as the angle ABC becomes > > 90° (Fig. S1b) and significantly shortens as angle ABC becomes < < 90° (not shown). Foreleg length was measured as the number of pixels between the neck indentation and the bottom of the proximate foreleg hoof. Hindleg length was measured between the tuber sacrale (the top of the croup) and the bottom of the hindleg hoof. In cases where the forelegs and hindlegs deviated substantially from perpendicular, the bottom of the hoof landmark was moved horizontally to a position that was nearly perpendicular. Neck width was measured between the neck indentation to the withers neck-bending inflection point. The girth of the neck (neck thickness) was not measured in this study but is known to be proportionally greater in adult males, in part due to the lateral increase of muscle mass (Simmons and Scheepers 1996; Simmons and Altwegg 2010; Mitchell 2021). By contrast, neck width is largely determined by the length of the scapula and dorsal spines of the anterior thoracic vertebrae (Mitchell 2021).
Calculation of body proportions
Relative body proportions of each giraffe were calculated either as a ratio of two body length measurements or calculated as a proportional fraction of potential vertical height (PVH) composed of the sum of the neck and foreleg lengths (Fig. 1). In addition, we summed the lengths of each of the components of the axial and appendicular skeleton as the total axial-appendicular length, and then the fractions of the neck, trunk, foreleg, and hindleg of the total axial-appendicular length for each individual were calculated. Because the axial:appendicular ratio is somewhat confounded by the large growth rate differences of the fore and hindlegs, we also compared growth of various skeletal components to the hindleg only, which shows the least variation between males and females in this study (Table S1).
Other giraffe studies that have compared relative neck to foreleg length using postmortem measurements (van Sittert et al. 2010; Mitchell 2021) measured the distance between the withers peak to the occipital ridge as neck length and the distance between the withers peak to the bottom of the foreleg hoof as foreleg length. However, the precise location of the withers peak is difficult to discern in photographs of most giraffes in contrast to the neck indentation. Consequently, we chose the neck indentation as the primary central point dividing the neck and foreleg lengths. To confirm that the use of the neck indentation as the central dividing point yielded similar results to using the withers peak, we also measured the relative neck and foreleg lengths by this alternative method estimating the location of the withers peak with uncertain accuracy.
To evaluate the statistical significance of male-female differences in body proportion, we used a mixed-effects analysis of covariance (ANCOVA) to model the sex-specific body proportions of 179 captive Masai giraffes as an outcome of sex and age. Sex was treated as a categorical variable, and age as a continuous covariate. Because the data included multiple images for each individual giraffe photographed at different ages, giraffe-level random effects are included in the model. By visual inspection, age and giraffe body proportion are not linearly related, so quadratic and cubic-order terms are also included.
We also compared the body proportions of adult captive giraffes to those of adult wild giraffes using a two-factor ANOVA with sex and wild/captive status as factors. Body proportion data for this analysis were collected from images of adult giraffes > 8 years of age. No repeated measurements were used.
In addition to measuring lengths of various body components, we measured the two-dimensional area of the neck and the hindquarters as proxies for relative mass of these two body components. FIJI Image J (Schindelin et al. 2012) was used to trace the outline of the neck and hindquarters (Fig. S4) and determine the pixel area. The fraction of neck area to the total area of neck and hindquarters for each individual was calculated and evaluated as a function of sex and location (captive or wild) by two-factor ANOVA.
The significance level (alpha) of all models were Benjamini-Hochberg (Benjamini and Hochberg 1995) corrected to mitigate the inflation of type 1 error (false positives) due to multiple testing. For all models, quantile–quantile plots suggest that the assumption of normality is fulfilled for all 12 parameters evaluated (Fig. S2, S3). For the two-factor ANOVAs, residual plots show that the errors are homoscedastic. The residual plots for the longitudinal ANCOVA analysis showed a slight departure from constant variance.
We observed that some individuals did not appear to display sex-appropriate body proportions. To evaluate this further, we used discriminant analysis of principal components (DAPC) (Jombart et al. 2010) to evaluate proportional neck, trunk, foreleg, and hindleg lengths compared to total axial-appendicular length; neck width to neck length ratio; and hindleg length to axial skeleton length (neck + trunk length) ratio for adult captive and wild Masai giraffes to define sex-specific morphology phenotypes. To quantitatively evaluate the degree to which each individual displayed sex-appropriate body proportions, we plotted the first discriminant function of the DAPC for each individual and determined their sex membership probability for male versus female body proportion phenotypes.
Results
Body proportions change as a function of developmental age and sex
We found that male and female captive Masai giraffe calves do not exhibit any body proportion difference at birth and during the first few years of postnatal development (Fig. 2). The axial:appendicular ratio steadily increased in both sexes during the first 8 years, indicating a faster growth rate of the axial skeleton (Table 1, Fig. 2a). No significant sex differences were observed in the axial:appendicular ratio during the first few years (Table 1, Fig. 2a), but by the eighth year females exhibited a significantly larger axial:appendicular ratio than males which persists in mature adults (Table 2, Fig. 3). Within the axial skeleton, the neck grows at a faster rate than the trunk (Table 1), but no sexual dimorphisms were seen in either the relative growth rates or the neck to trunk ratio in mature adults (Table 1, Fig. 2b). Within the appendicular skeleton the foreleg was found to grow at a faster pace than the hindleg (Table 1, Fig. 2c) in both sexes and was particularly high in males (Fig. 2c), which yielded a relatively large sexual dimorphism in foreleg to hindleg ratio in mature adults (Tables 2, 3). The growth of the foreleg in males compared to the growth of the trunk in females (Trunk/Foreleg ratio) illustrates the dichotomous trajectories of sexual dimorphisms in body proportions (Fig. 2d). Both sexes exhibit an increase in the trunk to foreleg ratio during the first five years, but after this age the male trajectory reverses as males experience an apparent surge in foreleg growth as they reach sexual maturity (Fig. 2d). By contrast females continue to expand their trunks relative to their forelegs through the tenth year (Fig. 2d).
Masai giraffe body proportions across the lifespan. Body proportions of 179 individuals were estimated from 357 images with 1–5 images per individual taken at different ages. Red = females; Blue = males. A linear mixed-effects model, with random effects for each individual, was used to generate the plots. Capped lines = 95% confidence interval. Vertical black lines and years (large font) indicate the first age class for which males and females are significantly different indicating a sex × age interaction effect. Excluding Neck/Trunk, all body proportions differ in slope, with the two lines diverging as age increases. However, none of the intercepts differ significantly as there are no body proportion differences in male and female calves during the first year. Axial/Append = Axial skeleton (neck length + trunk length)/Appendicular skeleton (foreleg length + hindleg length), Neck/Trunk = neck length/trunk length, Foreleg/Hindleg = foreleg length/hindleg length, Trunk/Foreleg = trunk length/foreleg length, %Neck = neck length /total potential anterior height (PVH) comprised of the neck and foreleg lengths, Axial/Hindleg = axial length/hindleg length, Axial/Foreleg = axial length/foreleg length, Neck/Hindleg = neck length/hindleg length, Trunk/Hindleg = trunk length hindleg length, wNeck/wForeleg = withers to occipital ridge/withers to foreleg hoof, WithH/RumpH = withers height/rump height, NeckW/NeckL = neck width at proximal base/neck length
Comparison of adult male and female body proportions of captive and wild Masai giraffes. Tukey comparisons of mean differences of body proportions for each of the sexes by location. Pairwise comparisons are plotted on the y axis along with 95% confidence intervals. For group pairs that cross the origin '0' line there is no significant difference between their body proportions. In the Tukey comparison performed the “family-wise” error rate was adjusted to account for multiple testing across body proportions and to account for multiple testing across pairs of groups. C = Captive, W = Wild, M = Male, F = Female
The potential vertical height (PVH) of giraffes is determined by the length of the forelegs and neck. We found that neck length in adult females comprised a larger fraction of PVH than in males (Table 2, Fig. 2e). The proportionally longer forelegs compared to hindlegs in adult males (Tables 2, 3) partly contributes to this finding. We speculated that the hindleg length may be the only measured component that does not differ proportionally between the sexes and may serve as an additional comparative metric. To test this hypothesis, we summed the four body lengths (neck, trunk, foreleg, and hindleg), calculated the fraction of each component to length sum, and evaluated sex differences (Table S1). As expected, we found that the neck and trunk contributed a larger fraction of the total in females, whereas the foreleg contributed a larger fraction in males (Table S1). However, the hindleg contribution to the total was not significantly different between the sexes. Using the hindleg length as a standard for comparison, we found that the axial component (neck + trunk lengths) to the hindleg exhibits a female-biased BpSD (Fig. 2f) consistent with the female bias in axial to appendicular (Fig. 2a) and axial to foreleg ratio (Fig. 2g). Comparing the individual axial components to hindleg length revealed that the neck to hindleg length ratio shows a small but insignificant female bias (Fig. 2h) whereas the trunk to hindleg length ratio shows a significant female bias (Fig. 2i).
We further measured the relative neck and foreleg lengths (wNeck/wForeleg) (Fig. 2j) using the withers peak as the central landmark for PVH as is typically measured in postmortem samples (Mitchell 2021). At birth, no sex differences were seen in wNeck/wForeleg (Fig. 2j), but by year 7, neck length contributes a proportionally larger fraction of PVH than the forelegs in females compared to males and yields a highly significant difference at the adult stage using this method (Tables 2, 3).
Body height in all other ungulates besides the giraffe is considered to be the height of the withers lying at the base of the neck above the shoulders (Thomas 2005). We measured the withers height proportional to the rump (croup) height (WithH/RumpH) and found no sexual dimorphism at birth. By the age of 4.5 years male giraffes have proportionally greater withers height than females (Fig. 2k) consistent with the foreleg to hindleg BpSD (Fig. 2c) which contribute to the largest fraction of withers and rump height, respectively.
We measured proximal neck width, the distance between the neck indentation and the withers peak, compared to neck length to determine if a BpSD exists for this trait. The neck width to length ratio was not significantly different between the sexes in newborn calves (Fig. 2l). Due to the relatively short neck length of newborn calves, the neck width: length ratio is highest in calves and then decreases as neck length rapidly grows (Fig. 2l). However, the progressive decrease in male neck width: length ratio ceases by the end of the second year in males but continues to decline in females until approximately the tenth year (Fig. 2l). As a result, the neck width to neck length ratio is much larger in adult males than adult females (Tables 2, 3).
Body proportion sexual dimorphisms in adult captive and wild giraffes
Mean proportional neck lengths of wild adult Masai giraffes were nearly identical to captive Masai giraffes and showed a highly significant longer neck proportion of the total potential height in females than males (Table 1). Wild Masai giraffes also displayed the same pattern of sex-specific differences for the other traits including proportionally longer trunks, necks, and axial skeletons in females and proportionally longer forelegs and wider necks in males (Tables 2, 3).
Because captive and wild females have proportionally longer necks, but males have proportionally wider necks, it prompts the question as to whether females or males have proportionally larger necks in mass. Although it is not possible to estimate mass from two-dimensional images, we can use the two-dimensional area of the neck as a proxy for mass assuming that the lateral growth to the body anterior–posterior axis is proportional to the area (Mitchell 2021). The body torso area would appear to be the most logical choice for comparing neck area. However, the body torso (trunk) is proportionally longer in females and can be greatly extended ventrally in females during pregnancy. Therefore, we chose the hindquarters area as a comparative variable (Fig. S4) because it appears to be proportionally equivalent in males and females (Table S1) and does not change during pregnancy. We found that neck area, compared to hindquarter area, is proportionally larger in males than females of both captive and wild adult giraffes (> 8 years of age) (Fig. 4).
Comparison of relative neck area in female and male adult, captive and wild, Masai giraffes. Ratios of neck area to hindquarter area are plotted on the y axis. The horizontal bold lines are the mean values, and the vertical lines represent data points within the 95% confidence interval. Females and males are highly significantly different for both captive and wild giraffes (p < 0.001) by ANOVA
The estimated mean lengths of the neck, trunk, and legs define G. tippelskirchi adult female body phenotypes (FBP) and adult male body phenotypes (MBP) (e.g., Fig. 5a–c). However, some giraffes of both sexes, and particularly males, exhibited opposite-sex phenotypes (e.g., Fig. 5b–d). To quantify sex-specific phenotypes, we performed a discriminate analysis of principal components (Jombart et al. 2010) of neck, foreleg, hindleg, and trunk lengths as fractions of the total axial-appendicular length, neck width to neck length ratio, and the axial to hindleg length ratio using female and male sex as the prior groups for both captive and wild giraffes. The initial principal component analysis (PCA) showed the expected closer correlation of the neck and trunk of the axial skeleton and the forelegs and hindlegs of the appendicular skeleton (Fig. S5). The DAPC analysis of the membership probability of each sex showed that the average probability of FBP for G. tippelskirchi was 93.1% and 92.9% for captive and wild individuals, respectively, and the average probability of MBP for G. tippelskirchi was 79.1% and 92.1% for captive and wild giraffes, respectively (Fig. 6 and Table S2). Only one captive female (1.9%) and one wild female (2.5%) exhibited greater than 50% MBP phenotype membership, whereas four captive males (15%) and three wild males (5.2%) exhibited greater than 50% FBP phenotype membership. The relative differences in sex phenotypes between captive and wild giraffes were also readily seen in the linear discriminant analysis (Fig. S6), which showed a broader range and greater overlap in captive giraffe FBP and MBP phenotypes. It is important to note that the fertility of captive giraffes exhibiting the most discordant body proportion phenotypes appeared to be normal. For example, only two captive males exhibited > 90% FBP membership probability, but they have both sired several offspring. However, these captive males had no competition for mating. By contrast, we do not know the relative mating success of wild males according to phenotype.
Examples of male and female Masai giraffe body proportion phenotypes. a Prototypical female giraffe displaying a female body proportion phenotype with long neck and trunk and shorter legs. b Female giraffe displaying a male body proportion phenotype with relatively short trunk and neck and longer legs. c Prototypical male giraffe displaying a male body proportion phenotype with shorter neck and trunk and longer legs. d Male giraffe displaying a female body proportion phenotype with longer neck and trunk and shorter legs, but still displaying male secondary sexual characteristics of the head (thick robust ossicones, secondary ossicones, and thick muscular neck)
Discriminate analysis of principal components (DAPC) of female and male body proportion phenotypes in captive and wild Masai giraffes. The relative body proportions of the neck, trunk, foreleg, and hindleg to the total axial-appendicular length, neck width to neck length ratio, and axial skeleton to hindleg length ratio were used as the input parameters for the DAPC. The first discriminant function of the DAPC shows distinct profile and peak differences between females and males of captive (a) and wild (b) giraffes but a large degree of overlap is seen in captive giraffes. Sex-specific body proportion membership probabilities for each individual for captive (c) and wild (d) giraffes. See Table S2 for numerical values of the sex-specific membership probabilities for each individual
Discussion
During postnatal development the various components of the axial and appendicular skeleton generally show positive allometry with body size in giraffes (Mitchell 2021). The components of the axial and appendicular skeleton exhibit a high degree of morphological integration as determined by shared developmental origins and functional integration (Hanot et al. 2017; Randau and Goswami 2017; Arlegi et al. 2020; Mallo et al. 2021). Within those constraints specific components can be modified for specialized functions such as the change in forelimb function of hominids from locomotion to grasping (Pouydebat et al. 2008; Stamos and Alemseged 2023) and the extension of the neck in giraffes (Mitchell 2021). However, the length of the neck grows proportionally faster than any other part of the body (Mitchell 2021) yielding a neck length that comprises the largest fraction of the axial skeleton of any existing mammal (Badlangana et al. 2009). These specialized adaptations of skeletal components require compensatory changes in other skeletal components to maintain overall functional integration. For example, to maintain balance and locomotion the evolutionary extension of the giraffe’s neck and legs required shortening the trunk and shifting the neck to be more posteriorly positioned above the forelegs (Mitchell 2021). Giraffes’ lofty stature is not only caused by their long neck but also by extending the legs and lengthening the scapula and thoracic dorsal spines compared to other artiodactyls. The morphological integration of these changes places considerable allometric constraints on individual variation. Nonetheless, we found significant sexual dimorphisms in axial and appendicular skeletal components in Masai giraffes. Specifically, adult females have proportionally longer necks and trunks, whereas males have proportionally longer forelegs and wider necks. These body proportion sexual dimorphisms (BpSD) are seen in both captive and wild adult Masai giraffes and their magnitudes are virtually identical. This finding supports the hypothesis that the BpSDs are largely, if not entirely, genetically determined and justifies the use of captive giraffes to interrogate postnatal development of these traits.
At birth we found no differences in body proportions between captive female and male calves. The growth rate of male calves is faster than females during the first year, but BpSDs do not become statistically significant until sometime after the third year when giraffes are beginning to reach sexual maturity. These BpSDs are therefore likely to be determined by hormonal differences between the sexes that arise during puberty in a similar manner to sexual dimorphisms in other species (Cox et al. 2009).
We speculate that the adaptive functions of the body proportion dimorphisms in Masai giraffes are threefold: (1) The extension of the axial skeleton in female giraffes serves to expand the browsing lateral range by lengthening the neck and to provide sufficient space for prenatal development by lengthening the body trunk. Observation of feeding behavior of female giraffes indicates that their long necks are advantageous in reaching deep into acacia thickets horizontally (Mitchell 2021) rather than reaching at the tops of trees. Thus, female giraffes, through the proportional extension of the axial skeleton, have increased the horizontal dimension to effect higher reproductive capacity. (2) The proportional extension of the forelegs and the increased withers height serve to enhance male mating competitiveness by increasing the vertical height of the anterior body trunk. (3) The increase in proximal neck width in males is correlated with proportionally larger neck area as shown herein and neck mass as shown by Simmons and Altwegg (2010). As a result, the proximal base of the neck is elevated, the neck mass is enhanced, and the forelegs are longer potentially providing increased leverage and force during neck-sparring competitions. Additionally, the slope of the back increases which accentuates the appearance of size and dominance. The shortening of the body trunk in males compared to females also further enhances this appearance. These BpSDs combined with the large body size sexual dimorphism result in an overall imposing stature of a mature adult male.
Male giraffes establish a dominance hierarchy by dominance display behavior and through physical contact with each other. Male-male physical contact includes body-pushing (e.g., shoulder-shoulder engagement) and neck-sparring behavior (Coe 1967; Pratt and Anderson 1985). Dominant males are usually but not always larger (Dagg and Foster 1976; Pratt and Anderson 1985), and we speculate that withers height and neck mass are likely to be more advantageous than longer necks. Once dominance relationships have been established, male giraffes remember and recognize males of higher rank by sight (Pratt and Anderson 1985). Most male-male interactions are low intensity involving behavioral displays, body pushing, and neck sparring (Coe 1967; Pratt and Anderson 1985). High-intensity neck-fighting is rare and usually involves individuals that are unknown to each other (Pratt and Anderson 1985). Whether females choose mates based upon size and appearance is unknown because copulation in the wild is rarely observed and paternity is never known.
We propose that the male-biased BpSDs are evolutionarily tied to the large male-biased body size sexual dimorphism (SSD) seen in giraffes. Male-biased SSD evolved primarily in ungulate species with comparatively large body size and that are polygynous, social, and live in open habitats (McPherson and Chenoweth 2012; Pérez-Barbería et al. 2002; Polák and Frynta 2009; Roylance-Casson 2021; Cameron and du Toit 2007). The presence of male-biased SSD in giraffes but not in Okapia johnstoni (Roylance-Casson 2021), the giraffe’s closest existing relative, is largely consistent with these trends. Giraffes are larger, more social, and live in open habitats, whereas okapis are exclusively found in closed canopy forests (Stanton et al. 2014). Male-biased SSD is argued to be the product of sexual selection particularly in polygynous species where all females will be reproductively successful but not all males will be (McPherson and Chenoweth 2012). Female giraffes begin reproduction as early as the third year and may continue to produce offspring throughout their lives. By contrast male giraffes have a much shorter window for reproduction because males are not successful in mating until they become large enough to outcompete other males, and their lifespan is approximately 25% shorter than females in captivity and in the wild (Bingaman Lacky and LaRue 1997; Berry and Bercovitch 2012; Bercovitch and Berry 2017). The much shorter lifespan of male giraffes is almost certainly due to the considerable cost to their skeletal health of having 30–40% greater body mass (Hall-Martin 1977; Mitchell 2021; Roylance-Casson 2021). The increase in body mass, with the largest fraction compressed on top of the forelegs, results in foreleg joint and hoof dysfunction as male giraffes reach 15 years of age. In addition to poorer skeletal health, adult males are less likely than females to survive severe droughts because they require proportionally more nutrition to survive (Mitchell et al. 2010). Mitchell (2021) has persuasively argued that giraffes, and particularly adult males, have pushed the limits of the skeletal system to withstand the gravitation force exerted by the mass of the anterior trunk, neck, and head stacked on top of their long, spindly forelegs. We speculate that the elevation of the forelegs and wither height, along with a proportionally shorter trunk in adult males as reported herein exacerbate this physical challenge by shifting additional body mass over the forelegs. Differential niche occupation has been proposed as a potential explanation for SSD in giraffes and other large herbivores (Ruckstuhl and Neuhaus 2002). Male and female giraffes do tend to browse at different levels and may select different types of browse (Ginnett and Demment 1999; Cameron and du Toit 2007; Mramba et al. 2017). However, the substantial cost of male giraffe stature to longevity and resilience argues against this hypothesis as the primary selective pressure that resulted in SSD. We propose that dominance competitions and access to mating are the major drivers of sexual selection for male-biased SSD and BpSD. For male giraffes, having an elevated anterior body trunk and appearing to be bigger is believed to determine the degree of reproductive success (Pratt and Anderson 1985), but it has yet to be supported with genetic evidence of reproductive success differences among male phenotypes.
While we favor sexual selection as the explanation for the male-biased size and body proportion sexual dimorphisms in giraffes, the preponderance of evidence supports the hypothesis that the giraffe’s long neck and tall stature initially evolved through natural selection by foraging competition with other ungulate browsers (Cameron and du Toit 2007; Wilkinson and Ruxton 2012) perhaps driven by the increased nutritional demands of female giraffes during gestation and lactation (Parker et al. 2009; Newby and DeCesare 2020). The female-biased BpSD, with the expanded neck and trunk shown herein, is consistent with the natural selection hypothesis.
A key prediction of the necks-for-sex hypothesis as originally proposed (Simmons and Scheepers 1996) is that male giraffes should have proportionally longer necks. However, Mitchell and coworkers (Mitchell et al. 2009, 2013; Mitchell 2021) have shown that for the South African giraffes (G. giraffa) females have proportionally longer necks than males. Our study on Masai giraffes (G. tippelskirchi) confirms their finding that adult females have proportionally longer necks than adult males. But in contrast to South African giraffes where males apparently have longer trunks (Mitchell et al. 2013; Mitchell 2021), we found that body trunks are proportionally longer in female Masai giraffes than males. In addition, we found that adult male giraffes have proportionally longer forelegs, consistent with the findings for South African giraffes as well as Angolan giraffes, G.g. angolensis (Silberbauer et al. 2021). In addition, we showed that the male neck is proportionally wider at the proximal base and that neck area, a proxy for mass, is proportionally larger in males likely due to an apparent increased muscle mass. These findings are germane to the modified necks-for-sex hypothesis (Simmons and Altwegg 2010) that emphasizes relative neck size and mass over neck length. Simmons and Altwegg (2010) report positive allometry of neck mass to overall body mass in Namibian male giraffes but isometry for females. By contrast, Mitchell (2021) and colleagues (Mitchell et al. 2009) report no sex difference in the relationship of neck mass to overall body mass for giraffes in Zimbabwe although Simmons and Altwegg (2010) disputed the statistical analyses of their data. Our finding that male Masai giraffes have proportionally larger necks than females, despite being proportionally shorter, is consistent with the hypothesis that sexual selection has played a role in the evolution of the giraffe’s neck. While we favor the competitive browse hypothesis as the original driver of the evolution of the giraffe’s long neck, we speculate that male-biased sexual selection may have later co-opted long necks as an exaptation as suggested by Simmons and Altwegg (2010).
The mean body proportion sex differences define stereotypical male and female giraffe phenotypes: females with long necks and trunks and males with long forelegs and wide necks. However, we discovered some individuals of both sexes that displayed opposite-sex body proportion phenotypes in both captive and wild giraffes. Captive males exhibited the largest fraction of discordant phenotypes. In the majority of these exceptional cases, a departure from expected sex phenotype was due to a difference in the axial to appendicular ratio, and not to just one part of the skeleton. Differential growth regulation of the giraffe’s axial and appendicular skeleton is suggested by the discovery and characterization of two wild giraffes that displayed disproportionate dwarfism characterized by shortened legs but normal neck and trunk (Brown and Wells 2020). Under this model, downstream axial and appendicular specific growth factors would need to regulate the additional expansion of neck in females and forelegs in males. That captive male giraffes exhibit a much higher sex phenotype discordancy suggests that the underlying selective forces may be relaxed in captivity. We postulate that sexual selection is the most obvious candidate for maintaining these BpSDs in the wild, whereas mating in captivity is entirely arranged. Individuals exhibiting opposite-sex body proportion phenotypes may be indicative of an underlying parental competition for growth control which is relaxed in captivity. Several growth control genes in humans and other mammals have been discovered to be genomically imprinted such that either the maternal or paternal allele is repressed (Bartolomei and Tilghman 1997; Wu et al. 2004; Ishida and Moore 2013). Moreover, the balance in parental competition for growth control can also be impacted by nutrition through epigenetic mechanisms. Further studies are needed to identify the molecular, developmental, and environmental factors that determine sexual dimorphisms in giraffe body proportions.
Data accessibility statement
Data used in this manuscript including giraffe images, body measurements, and body proportion calculations are deposited in ScholarSphere: Cavener, Douglas, et al. (2023), Masai Giraffe Sexual Dimorphisms in Body proportions doi:https://doi.org/10.26207/45ta-gp57.
References
Abouheif E, Fairbairn DJ (1997) A comparative analysis of allometry for sexual size dimorphism: assessing Rensch’s rule. Am Nat 149:540–562. https://doi.org/10.1086/286004
Arlegi M, Veschambre-Couture C, Gómez-Olivencia A (2020) Evolutionary selection and morphological integration in the vertebral column of modern humans. Am J Phys Anthropol 171:17–36. https://doi.org/10.1002/AJPA.23950
AZA (2023) American Association of Zoos and Aquariums: species survival plan programs. https://www.aza.org/species-survival-plan-programs. Accessed 20 May 2023
Badlangana NL, Adams JW, Manger PR (2009) The giraffe (Giraffa camelopardalis) cervical vertebral column: a heuristic example in understanding evolutionary processes? Zool J Linn Soc 155:736–757. https://doi.org/10.1111/j.1096-3642.2008.00458.x
Badyaev AV, Hill GE, Whittingham LA (2001) The evolution of sexual size dimorphism in the house finch IV. Population divergence in ontogeny. Evolution 55:2534–2549. https://doi.org/10.1111/j.0014-3820.2001.tb00767.x
Bartolomei MS, Tilghman SM (1997) Genomic imprinting in mammals. Annu Rev Genet 31:493–525. https://doi.org/10.1146/annurev.genet.31.1.493
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Bercovitch FB, Berry PSM (2017) Life expectancy, maximum longevity and lifetime reproductive success in female Thornicroft’s giraffe in Zambia. Afr J Ecol 55:443–450. https://doi.org/10.1111/aje.12370
Berry PSM, Bercovitch FB (2012) Darkening coat colour reveals life history and life expectancy of male Thornicroft’s giraffes. J Zool 287:157–160. https://doi.org/10.1111/j.1469-7998.2012.00904.x
Bingaman Lacky L, LaRue F (1997) North American regional giraffe studbook. Int Spec Inf Syst, Dallas
Bond ML, Lee DE, Ozgul A et al (2021) Leaving by staying: Social dispersal in giraffes. Anim Ecol 90:2755–2766. https://doi.org/10.1111/1365-2656.13582
Brown MB, Wells E (2020) Skeletal dysplasia-like syndromes in wild giraffe. BMC Res Notes 13:569. https://doi.org/10.1186/s13104-020-05403-9
Cameron EZ, du Toit JT (2007) Winning by a neck: Tall giraffes avoid competing with shorter browsers. Am Nat 169:130–135. https://doi.org/10.1086/509940
Cantwell E (2018) AZA regional studbook giraffe Giraffa camelopardalis. Mar Zoo, Baltimore
Cassini MH (2020) A mixed model of the evolution of polygyny and sexual size dimorphism in mammals. Mamm Rev 50:112–120. https://doi.org/10.1111/mam.12171
Clutton-Brock TH (1989) Review lecture: mammalian mating systems. Proc R Soc Lond B Biol Sci 236:339–372. https://doi.org/10.1098/rspb.1989.0027
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365. https://doi.org/10.1006/anbe.1995.0166
Coe MJ (1967) “Necking” behaviour in the giraffe. J Zool 151:313–321. https://doi.org/10.1111/J.1469-7998.1967.TB02117.X
Cox RM, Stenquist DS, Calsbeek R (2009) Testosterone, growth and the evolution of sexual size dimorphism. J Evol Biol 22:1586–1598. https://doi.org/10.1111/J.1420-9101.2009.01772.X
Dagg AI, Foster JB (1976) The Giraffe: its biology, behavior, and ecology. Van Nostrand Reinhold, New York
Darwin C (1871) The descent of man and selection in relation to sex. John Murray, London
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Foster JB (1966) The giraffe of Nairobi National Park: home range, sex ratios, the herd, and food. Afr J Ecol 4:139–148. https://doi.org/10.1111/j.1365-2028.1966.tb00889.x
Ginnett TF, Demment MW (1999) Sexual segregation by Masai giraffes at two spatial scales. Afr J Ecol 37:93–106. https://doi.org/10.1046/j.1365-2028.1999.00163.x
Hall-Martin AJ (1977) Giraffe weight estimation using dissected leg weight and body measurements. J Wildl Manage 41:740. https://doi.org/10.2307/3799999
Hanot P, Herrel A, Guintard C, Cornette R (2017) Morphological integration in the appendicular skeleton of two domestic taxa: the horse and donkey. Proc R Soc Lond B Biol Sci 284:20171241. https://doi.org/10.1098/rspb.2017.1241
Ishida M, Moore GE (2013) The role of imprinted genes in humans. Mol Aspects Med 34:826–840
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94. https://doi.org/10.1186/1471-2156-11-94
Lee DE, Bond ML, Kissui BM et al (2016) Spatial variation in giraffe demography: a test of 2 paradigms. J Mammal 97:1015–1025. https://doi.org/10.1093/jmammal/gyw086
Leutenegger W, Cheverud J (1982) Correlates of sexual dimorphism in primates: ecological and size variables. Int J Primatol 3:387–402. https://doi.org/10.1007/BF02693740
Lohay GG, Lee DE, Wu-Cavener L et al (2023) Genetic evidence of population subdivision among Masai giraffes separated by the Gregory Rift Valley in Tanzania. Ecol Evol 13:e10160. https://doi.org/10.1002/ece3.10160
Mallo M, Buffetaut E, Diaz RE (2021) Of necks, trunks and tails: axial skeletal diversity among vertebrates. Diversity 13:289. https://doi.org/10.3390/d13070289
McPherson FJ, Chenoweth PJ (2012) Mammalian sexual dimorphism. Anim Repro Sci 131:109–122. https://doi.org/10.1016/j.anireprosci.2012.02.007
Mitchell G (2021) How giraffes work. Oxford University Press eBooks. https://doi.org/10.1093/oso/9780197571194.001.0001
Mitchell G, Van Sittert SJ, Skinner JD (2009) Sexual selection is not the origin of long necks in giraffes. J Zool 278:281–286. https://doi.org/10.1111/j.1469-7998.2009.00573.x
Mitchell G, van Sittert S, Skinner JD (2010) The demography of giraffe deaths in a drought. Trans R S S Afr 65:165–168. https://doi.org/10.1080/0035919X.2010.509153
Mitchell G, Roberts D, van Sittert S, Skinner JD (2013) Growth patterns and masses of the heads and necks of male and female giraffes. J Zool 290:49–57. https://doi.org/10.1111/jzo.12013
Mramba RP, Mahenya O, Siyaya A et al (2017) Sexual segregation in foraging giraffe. Acta Oecol 79:26–35. https://doi.org/10.1016/J.ACTAO.2016.12.007
Newby JR, DeCesare NJ (2020) Multiple nutritional currencies shape pregnancy in a large herbivore. Can J Zool 98:307–315. https://doi.org/10.1139/cjz-2019-0241
Parker KL, Barboza PS, Gillingham MP (2009) Nutrition integrates environmental responses of ungulates. Funct Ecol 23:57–69. https://doi.org/10.1111/j.1365-2435.2009.01528.x
Pérez-Barbería FJ, Gordon IJ, Pagel M (2002) The origins of sexual dimorphism in body size in ungulates. Evolution 56:1276–1285. https://doi.org/10.1111/j.0014-3820.2002.tb01438.x
Polák J, Frynta D (2009) Sexual size dimorphism in domestic goats, sheep, and their wild relatives. Biol J Linnean Soc 98:872–883. https://doi.org/10.1111/j.1095-8312.2009.01294.x
Pouydebat E, Laurin M, Gorce P, Bels V (2008) Evolution of grasping among anthropoids. J Evol Biol 21:1732–1743. https://doi.org/10.1111/j.1420-9101.2008.01582.x
Pratt DM, Anderson VH (1985) Giraffe social behaviour. J Nat Hist 19:771–781. https://doi.org/10.1080/00222938500770471
Ralls K (1977) Sexual dimorphism in mammals: avian models and unanswered questions. Am Nat 111:917–938. https://doi.org/10.1086/283223
Randau M, Goswami A (2017) Morphological modularity in the vertebral column of Felidae (Mammalia, Carnivora). BMC Evol Biol 17:133. https://doi.org/10.1186/s12862-017-0975-2
Reiss MJ (1989) The allometry of growth and reproduction. Cambridge University Press, Cambridge
Rensch B (1959) Evolution above the species level. Columbia University Press, New York
Roylance-Casson E (2021) Rensch’s rule and the drivers of sexual dimorphism in ungulates. Bangor University, Bangor
Ruckstuhl KE, Neuhaus P (2002) Sexual segregation in ungulates: a comparative test of three hypotheses. Biol Rev 77:77–96. https://doi.org/10.1017/S1464793101005814
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Silberbauer BL, Strydom PE, Hoffman LC (2021) An exploratory study into the influence of sex on body measurements, carcass weights and meat yields of giraffe (Giraffa camelopardalis angolensis). Foods 10:2245. https://doi.org/10.3390/foods10102245
Simmons RE, Altwegg R (2010) Necks-for-sex or competing browsers? A critique of ideas on the evolution of giraffe. J Zool 282:6–12. https://doi.org/10.1111/j.1469-7998.2010.00711.x
Simmons RE, Scheepers L (1996) Winning by a neck: sexual selection in the evolution of giraffe. Am Nat 148:771–786. https://doi.org/10.1086/285955
Slatkin M (1984) Ecological causes of sexual dimorphism. Evolution 38:622. https://doi.org/10.2307/2408711
Stamos PA, Alemseged Z (2023) Hominin locomotion and evolution in the Late Miocene to Late Pliocene. J Hum Evol 178:103332. https://doi.org/10.1016/J.JHEVOL.2023.103332
Stanton DWG, Hart J, Galbusera P et al (2014) Distinct and diverse: range-wide phylogeography reveals ancient lineages and high genetic variation in the endangered Okapi (Okapia johnstoni). PLoS ONE 9:101081. https://doi.org/10.1371/JOURNAL.PONE.0101081
Strauss MLK (2014) Ecological and anthropogenic drivers of giraffe (Giraffa camelopardalis tippelskirchi) population dynamics in the Serengeti. PhD Dissertation, University of Minnesota
Tarnawski BA, Cassini GH, Flores DA (2014) Skull allometry and sexual dimorphism in the ontogeny of the southern elephant seal (Mirounga leonina). Can J Zool 92:19–31. https://doi.org/10.1139/cjz-2013-0106
Thomas HS (2005) The horse conformation handbook. Storey Publishing, North Adams
van Sittert SJ, Skinner JD, Mitchell G (2010) From fetus to adult-an allometric analysis of the giraffe vertebral column. J Exp Zool B Mol Dev Evol 314 B:469–479. https://doi.org/10.1002/jez.b.21353
Weckerly FW (1998) Sexual-size dimorphism: influence of mass and mating systems in the most dimorphic mammals. J Mammal 79:33–52. https://doi.org/10.2307/1382840
Wilkinson DM, Ruxton GD (2012) Understanding selection for long necks in different taxa. Biol Rev 87:616–630. https://doi.org/10.1111/j.1469-185X.2011.00212.x
Wu G, Bazer FW, Cudd TA et al (2004) Maternal nutrition and fetal development. J Nutr 134:2169–2172. https://doi.org/10.1093/jn/134.9.2169
Acknowledgements
Rebecca Bourne, Penn State University; James Madeli and Emmanuel Kimaro, Wild Nature Institute; Petra Campbell; Andrea Putnam, AZA and San Diego Zoo Global; Sheri Horiszyny AZA and Oregon Zoo; Heather Robertson, Nashville Zoo; Zoli Gyimesi, Louisville Zoo; Terri Roth, Cincinnati Zoo; Noah Dunham, Cleveland Zoo; Adam Felts, Columbus Zoo; Chris McKinney and Louis DiVincenti, Seneca Park Zoo; Louis Perrotti, Roger Williams Park Zoo; Lauren Hinton, Brevard Zoo; Chris Hamlin, San Diego Zoo; Judi Barnes and Cressa Nursement, Santa Barbara Zoo; Michele Green and Vernon Presley, Fresno Chaffe Zoo; Melissa McCartney, Sacramento Zoo. Wild giraffe photographic data collection in Tanzania supported by the Living Desert Zoo and Gardens, Sacramento Zoo, Tierpark Berlin, Tulsa Zoo, Columbus Zoo, Zoo Miami, Cincinnati Zoo, Toronto Zoo, Como Park Zoo, Roger Williams Park Zoo, and Save the Giraffes.
Funding
Funding were provide by Pennsylvania State University, Wild Nature Institute, and Huck Institutes of the Life Sciences.
Author information
Authors and Affiliations
Contributions
DRC: Conceptualization; data curation; statistical data analysis; methodology; writing—original draft and editing. MLB: data curation (supporting); writing—review and editing; LWC: data curation (supporting); writing—review and editing; GGL: data curation (supporting); writing—review and editing; MWC: data statistical analysis (primary); writing – review and editing. XH: data curation (supporting); writing—review and editing; DLP: statistical data analysis (supporting); writing—review and editing; DEL: data curation (supporting); methodology (supporting); writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Gertrud Rößner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cavener, D.R., Bond, M.L., Wu-Cavener, L. et al. Sexual dimorphisms in body proportions of Masai giraffes and the evolution of the giraffe’s neck. Mamm Biol (2024). https://doi.org/10.1007/s42991-024-00424-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42991-024-00424-4









