Abstract
Removal of zinc and cadmium from highly saline solutions by hydroxide precipitation is discussed. Experimental solubilities of zinc and cadmium in highly saline solutions were compared to modeled results obtained using Pitzer’s approach. An amphoteric character of zinc and cadmium and an influence of chloride ion on the concentration of dissolved metals were investigated. In order to avoid errors linked to pH measurements in concentrated aqueous solutions, the method of calibration of glass pH electrodes was developed and evaluated. The method uses easily prepared buffers whose pHs were determined with the Pitzer ion-interaction approach. The presented investigations address two issues of high significance in industrial wastewater treatment, namely: precise pH measurements and rigorous modeling of highly saline wastewaters. The results can be implemented in the treatment of hydrometallurgical wastewaters such as zinc refinery wastewater. Additionally, an implementation of the presented investigations is not limited to wastewater treatment but can easily be extended to other high-chloride metallurgical processes wherein the pH measurements in highly saline streams are required.
Graphical Abstract
Similar content being viewed by others
Introduction
The continuous progress of industrialization significantly affects the environment by contamination with heavy metals [1, 2]. Harmful effects of heavy metals contamination against living organisms are well known and well documented. To address this significant aspect, several methods of removal of heavy metals from wastewater have been developed but precipitation of metals remains the most popular technique on an industrial scale. The biggest advantages of the precipitation of the heavy metals are low cost and relative simplicity [3, 4]. Lime or caustic precipitation is particularly suitable for wastewaters containing high heavy metals content, e.g., electroplating or metallurgical wastewaters. Despite tremendous work done to optimize precipitation processes, there are still several challenges present. One such challenge is that heavy metals precipitation forms highly saline wastewaters.
The mobility of heavy metals depends on their binding forms and the different chemical species and minerals in which the metals prevail. The high concentration of background salts in wastewaters affects the solubility of heavy metals. For example, in hydrometallurgical wastewater such as the zinc refinery wastewater, the chloride ion is frequently present in concentration over 10,000 mg dm−3 [5]. Such a high concentration of chloride causes the formation of soluble complex chlorides of heavy metals. In fact, this phenomenon is so efficient that it is often used as a method to extract heavy metals from residues—brine leaching [6]. Unfortunately, the high solubility of heavy metals in highly saline solutions, being an advantage in the case of brine leaching, is a significant drawback in wastewater treatment [7, 8].
The quality of treatment by chemical precipitation depends strongly on the precision of the pH control, which in turn depends on the quality of pH measurements and dosing of chemicals. The pH measurements are the straightforward and frequent measurement performed in the wastewater industry. Typically, standard combined glass electrodes are utilized in both laboratory and industrial practice as pH measuring equipment. The combined glass electrode pH measurements are easy in use and precise equipment, however, several precautions have to be taken to guarantee the highest quality of measurements. The latest IUPAC Recommendations concerning pH measurements [9] describe the detailed procedure of pH measurements using glass electrodes and present limitations of their usage. The authors stress that pH measurements using pH meters calibrated on standard buffers are limited to solutions of low ionic strength (I < 0.1 mol kg−1). This aspect is not important when dealing with diluted wastewater streams, but its importance rises when, for example, the zinc refinery wastewater treatment is considered. The same drawbacks occur in other high-chloride processes wherein precipitation and pH measurements are required, e.g., HydroCopper™ technology, aimed to produce copper directly from concentrates. In the HydroCopper™ process, the copper concentrate is leached into a strong (250–300 g dm−3) sodium chloride solution and in the next phases, divalent copper is precipitated as hydroxychloride by increasing the pH of the solution to 4–5 with sodium hydroxide and Zn, Pb, Ni, etc., are removed as carbonates by increasing the pH to 6–7 using sodium carbonate [10]. Another process, wherein precipitation in highly loaded solutions has to be conducted, is Outotec Nickel Matte Chloride Leaching Process. The process incorporates metals leaching into a calcium chloride solution and subsequent iron precipitation using slaked lime or limestone [11].
Currently, there is no common and standardized convention describing recommendations on how to measure pH values in electrolyte solutions of moderate and high ionic strength. The main inconsistencies in pH measurements of high ionic strength solutions rise from the convention of assigning pH values to buffers. The assigned pH values for buffers, in line with IUPAC recommendations, are obtained using the Bates–Guggenheim convention and are only valid for dilute (I < 0.1 mol kg−1) solutions [12]. The Pitzer’s approach to ion interactions is widely appraised and offers an alternative to the Bates–Guggenheim suitable for use in solutions of higher ionic strengths [13].
The aim of this study is to introduce a possibility of using Pitzer’s approach to estimate the composition of simple buffers of high ionic strength and to model the precipitation of heavy metals from high ionic strength solutions. Further, the theoretical calculations are compared with the zinc and cadmium precipitation experiments, conducted in concentrated NaCl solutions. During the experimental works, the pH meter was calibrated against high ionic strength buffers, established using the above approach.
Experimental
Chemicals
Zinc(II) chloride (ZnCl2, Chempur, Poland) and cadmium(II) chloride hemi(pentahydrate) (CdCl2·2.5H2O, Chempur, Poland) were used to prepare heavy metal solutions. The salinity of the solutions was increased by adding sodium chloride (NaCl, Avantor, Poland). The solutions were prepared by dissolving the chemicals with distilled water. The standard pH buffers at pH 4.0 (citrate) and 9.0 (borate) for pH meter calibration were purchased from Chempur, Poland. 1 M NaOH, 1 M HCl (Chempur, Poland) standard solutions, and sodium bicarbonate (NaHCO3, Chempur, Poland) were used to prepare high ionic strength buffers. The ionic strength of the buffers was fixed using sodium chloride (NaCl, Avantor, Poland). All chemicals were of analytical grade.
pH Measurements
Solution pH was measured with a combined, refillable glass electrode (Elmetron ERH-11, filling solution of a reference electrode: 3 M KCl + sat. AgCl, reference half-cell: Ag/AgCl) and an Elmetron CPC-461 pH meter. Purchased, commercial secondary standard buffers [9] at pH 4.0 (citrate) and 9.0 (borate) were used to calibrate the meter for pH measurements in the ISO scale [14]. In the case of highly saline solution pH measurements, the meter was calibrated against high ionic strength buffers (molality ~ 1.0 m) prepared in the laboratory. The composition of the high ionic strength buffers was established using PHREEQC software as described in the subsequent chapter.
In order to distinguish whether the standard buffers or the high ionic strength buffers were used to calibrate the meter, the following notation is used within the article: pHISO—depicts measurements carried out using meter calibrated against secondary standard buffers and pHHSW—using the high ionic strength buffers. The potential difference and temperature measured by the pH meter during calibrations were recorded and further used to convert to pH using a two-point calibration procedure, in line with IUPAC Recommendations [9].
The calibration of the pH meter was validated against two test solutions: acidic and alkaline. The solutions consisted of HCl, and NaOH + NaHCO3, respectively. The ionic strength of the solutions was changed by the cumulative addition of weighted portions of solid NaCl. The potential difference and temperature were recorded during NaCl addition.
Sample measurements and calibrations were carried out in jacketed glass beakers (100 ml and 250 ml). The temperature of water conveyed in the beakers’ jacket was held constant using the Julabo F25-HE thermostat.
Software Calculations
High Ionic Strength Buffers Calculations
In order to evaluate the theoretical pH of the prepared high ionic strength buffers, PHREEQC modeling software was used. The PHREEQC software is a computer program for speciation, batch reaction, one-dimensional transport, and inverse geochemical calculations [15]. The software offers several approaches to address electrolyte solution thermodynamics, among others: Debye–Hückel, Davies, Truesdell–Jones, SIT—implements extension of the Debye–Hückel theory introduced by Brønsted [16], WATEQ4F—uses extended Debye–Hückel equation [17], and Pitzer. The latter is especially suitable in the case of modeling of high ionic strength solution speciation [18] and therefore this approach was chosen.
The Pitzer’s model implementation in the PHREEQC is based on PHRQPITZ computer program [19] and excludes Pitzer interaction coefficients for several chemicals used in standard buffers (for example, phthalic acid, acetic acid, citric acid, or phosphoric acid). In order to avoid the necessity of manual addition of missing interaction coefficients, the prepared buffers were composed of constituents included in the default PHREEQC Pitzer’s database, namely: sodium hydroxide, sodium bicarbonate, sodium chloride, and hydrochloric acid. Such an approach is beneficial due to the usage of only high quality and well-documented Pitzer interaction parameters of sodium, chloride, or carbonate ions. In fact, a lack of Pitzer’s coefficients for relevant buffer media is claimed to be the main obstacle in the application of Pitzer’s model in pH measurements [13].
Subsequent advantage of using simple inorganic substances as buffer components is extending the shelf life of the buffers. Such high ionic strength buffers have a shelf life of at least one year when stored properly.
Zinc and Cadmium Solubility Predictions
The solubility of zinc and cadmium was also calculated using package PHREEQC and Pitzer’s approach [19]. In contrast to calculations of the high ionic strength buffers described above, zinc and cadmium solubility predictions required an extension of the default PHREEQC Pitzer’s ion-interaction parameters database. Contrary to components of the standard buffers, such data for cadmium and zinc chlorides are available in the literature. Implementation of the new species involved adding their pure-electrolyte and mixture interaction parameters. Interaction Pitzer parameters for Cd2+ and Zn2+ systems, together with its literature sources, are reported in Tables 1 and 2.
Anstiss and Pitzer [23] have pointed that in the case of CdCl2 there is some error in the value of ion-interaction parameters of Kim and Frederick [20]. Despite that, it was decided to include the parameters in the database because of their usage by Wang et al. [22] in establishing ternary mixing interaction parameters for NaCl–CdCl2–H2O system. Such an approach ensures that all interaction parameters are compatible [24].
PHREEQC Pitzer’s database was also constrained to include only those solid phases that were found to govern solubility. In the case of Cd2+ and Zn2+ dissolved in solutions containing high chlorides concentration, apart from hydroxides, zinc and cadmium hydroxy chlorides, can be formed during hydroxide precipitation [25, 26]. Therefore, in the case of NaCl–ZnCl2–H2O system considering Zn5(OH)8Cl2 precipitate [27, 28], and in the case of NaCl–CdCl2–H2O system CdOHCl are valid [29]. Thermodynamic constants for both aqueous and solid phases were selected from the PHREEQC databases for their relevance to the systems under study.
Precipitation Experiments
In order to check the solubility of cadmium and zinc in highly saline solutions and to compare experimental results with the model, the following solutions were prepared: ZnCl2 + NaCl (20 mmol kg−1water + 1300 mmol kg−1water, respectively) and CdCl2 + NaCl (30 mmol kg−1water + 1300 mmol kg−1water, respectively).
For each metal solution, ten 50 mL polypropylene vials were filled with approx. 45 g of the solution. Further, the vials were made up with 1 M NaOH ensuring heavy metal (zinc or cadmium) to hydroxide mole ratio ranging from 0 to 2.5. The addition of 1 M NaOH caused the immediate occurrence of white flocs. The vials were vigorously shaken to mix the constituents and left for 30 min. Next, the solutions were filtered using cellulose paper filters. The filtrate was collected and its pHHSW was measured. The concentrations of zinc and cadmium in the filtrate were determined on the iCAP 6500 Duo (Thermo Scientific, USA) inductively coupled plasma optical emission spectrometer (ICP-OES) according to EN ISO 11885:2009 “Water quality—determination of selected elements” by inductively coupled optical emission spectrometry (ICP-OES). To calibrate the spectrometer, calibration standard solutions were prepared from single standard element solutions from SCP Science Company with the addition of nitric acid.
The same procedure was used for both metals, namely zinc and cadmium, resulting in the obtention of 20 samples.
Results and Discussion
pH Meter Calibration
It was decided to calibrate the pH meter by a two-point calibration procedure using two buffer solutions. Such a procedure is recommended for the majority of glass electrode practical applications [9]. In such a case, the unknown pH(X) is obtained from the equation:
where the practical slope factor (k′) is given by
and pH(S1) and pH(S2) are pH values of standard buffers and EV(X), EV(S1), EV(S2) are respective potential differences measured. The IUPAC Recommendations claim that target uncertainty in pH(X) calibrated by a two-point calibration procedure should not exceed 0.03 in the case of usage of standard buffer solutions with an uncertainty 0.01. The overall uncertainty becomes higher if buffers with lower uncertainties are used.
In the case of the pHISO calibration, the pH meter was calibrated by measuring potential differences of two secondary standard buffers at 20 °C in accordance with the manufacturer’s guidelines and IUPAC Recommendations [9]. Buffers data and measured potential differences are summarized in Table 3.
The procedure of calibration in high ionic strength buffers (pHHSW calibration) was similar to above. The high ionic strength buffers were prepared individually. Bearing in mind the limitations of the Pitzer’s database in PHREEQC, the following buffer solutions were used: NaCl + HCl (pH 2.0 at 20 °C) and NaHCO3 + NaOH + NaCl (pH 10.0 at 20 °C). The composition of the buffers is adapted from Robinson and Stokes [30], but with the difference that NaCl is added to increase the ionic strength to 1.0 m. The amounts of constituents in the buffer solutions were determined by PHREEQC simulations.
PHREEQC offers extreme flexibility in calculations and allows, among others, determination of the amount of reagent necessary to maintain selected pH. This procedure was used to calculate high ionic strength buffers composition. In the case of the acidic buffer, PHREEQC was programmed to calculate the amount of the 1 M HCl necessary to fix the pH of the 1 m NaCl solution to 2.0. Similarly, in the case of the alkaline buffer, the amount of 1 M NaOH necessary to fix the pH of the 1 m NaCl + NaHCO3 solution to 10.0 was established. Further, the pH of the buffers at temperatures from 10 to 40 °C was calculated. The simulation source files used to calculate both buffer compositions and properties are included in the electronic supplementary material that accompanies this article. The results of the simulations are shown in Tables 4 and 5.
Two buffer solutions were prepared, in accordance with Table 4 and the pH meter was calibrated against them using the manufacturer’s guidelines. The calibration procedure was the same as in the case of calibration against the standard, diluted DIN buffers.
In order to validate the calibration of the meter, pH of two test solutions (acidic and alkaline) was recorded while changing the ionic strength, by addition of weighted portions of solid NaCl. A measured potential difference was converted to pH scale using two calibrations—using standard buffers (ISO scale) and high ionic strength buffers (HSW scale). Experimental data were compared with the theoretical pH of the solutions, calculated with PHREEQC software, using Pitzer’s approach. Calculated and measured pH of acidic and alkaline samples are shown in Figs. 1 and 2, respectively.
The gray band, shown in Fig. 1 (right) and Fig. 2 (right), represents the uncertainty of the procedure. The uncertainty of the procedure, as well as the uncertainty of measurement (depicted as vertical error bars), was estimated to ΔpH ± 0.03, likewise in the IUPAC Recommendations for pH measurements [9]. As shown in the figures, the experimental measurements obtained by pH meter calibrated using the ISO scale, fall into error limits for low ionic strength, which is consistent with the IUPAC Recommendations. The Bates–Guggenheim convention, used for the pH meter ISO calibration (as described in the recommendations), permits the applicability of the ISO procedure for ionic strength up to 0.1 mol kg–1. It can be seen that ISO calibration correlates pH fairly well for higher ionic strength, up to 0.5 mol kg–1.
However, further increase of the ionic strength makes the ISO-calibrated pH measurements unreliable. Unlike, the performance of HSW-calibrated pH is satisfactory for the higher ionic strength. The utility of high ionic strength buffer pH meter calibration is thus proved.
The result of the validation is a confirmation of purposefulness of using high ionic strength buffers for calibration of the pH meter when the examined solution has high ionic strength. The devised pH measurement methodology was used in further research on the solubility of zinc and cadmium in highly saline solutions, outlined below.
Zinc System
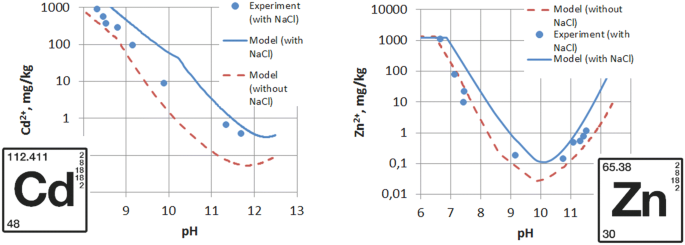
A comparison of the generated solubility and the experimentally observed precipitation profile for the zinc system is given in Fig. 3. The plot contains modeled solubility of zinc in solutions containing only zinc chloride (dashed line) and having additionally the high amount of NaCl (1300 mmol kg−1water, identical as in the experimental sample). It is clearly visible that a high concentration of Cl– anion causes an increase in zinc solubility. The minimum solubility is observed at pH 10.0. The Cl– concentration lowers pH of the minimal zinc solubility to some extent, reducing the optimal value. A typical amphoteric solubility relationship exists for zinc systems with and without the addition of the NaCl, with the onset of precipitation occurring at pH 7–9 and resolubilization occurring at pHs > 10.
A variation in the calculated and experimentally observed solubilities of zinc is visible, especially in lower pH region. This phenomenon may be explained by considering the kinetics of the precipitation. An analysis presented by Cousy et al. [26] shows that in the case of Zn solutions containing a high concentration of Cl– anion, a zinc hydroxide salt Zn5(OH)8Cl2·H2O—simonkolleite—is dominating component of the precipitates in the lower pH regions, while ZnO/Zn(OH)2 is present in a higher extent at pHs > 10. Further, the relation in the amount of both precipitates changes with time. Takada et al. subjected to aging for up to 200-h suspensions of ZnCl2 and NaOH and proved that the composition of precipitates changes over that time [27]. Taking into account that reaching equilibrium in such systems is prolonged, we can assume that the experimental samples shown in Fig. 3, subjected to aging for 30 min, have not reached final, equilibrium solubility. Despite the variation in the calculated and experimentally observed solubilities shown in Fig. 3, the increase in solubility of zinc caused by the high concentration of chloride ion is clearly visible.
PHREEQC simulations were used to quantify speciation of zinc systems containing no additional chlorides source and with a high concentration of NaCl. A comparison of speciations at different chlorides concentration as a function of pH is shown in Fig. 4. Figure 4 (left) shows that ZnCl2 solution without background NaCl contains mainly Zn2+ cation and [ZnCl]+ chloride complex in a minor extent. An increase of pH in the solution causes precipitation of Zn5(OH)8Cl2 in a narrow pH range from 6.5 to 7.2. Further increase of pH favors precipitation of ZnO. Above pH 12.5, the ZnO precipitate is being dissolved as a hydroxide complex anion [Zn(OH)4]2−. Speciation of ZnCl2 with background NaCl at a concentration of 1300 mmol kg−1water, shown in Fig. 4 (right), is more complex. At lower pH, complexed forms of zinc are dominant of which [ZnCl4]2− anion is prevailing, while free Zn2+ cation is almost absent. The addition of NaOH (an increase of pH) causes precipitation Zn5(OH)8Cl2 and its pH range is much wider than in the case of the solution without background NaCl. In solution having background NaCl, precipitation occurs at higher pH comparing to pure ZnCl2 solution. In the former solution, precipitation is observed at pH 6.8, while in the latter at pH 6.5. In the solution having a high concentration of background salt, a change in the precipitate composition from Zn5(OH)8Cl2 to ZnO occurs at much higher pH 8.8 comparing to 7.2 in the pure ZnCl2 solution. Further, the resolubilization of precipitate as a hydroxide complex occurs at lower pH 11.5 and is much prominent than in the case of pure ZnCl2 solution. Presented speciation analysis proves that chlorides and hydroxides present in a high concentration in zinc solutions affect both either speciation of solution and composition of precipitates. The relevance of the various hydroxy and chloro species distributions becomes evident in predicting the degree of solubility of the zinc precipitates. The shown analysis is in line with the calculations of Hahne and Kroontje [8] and extends mentioned work with precipitates composition.
A significant strength of chloride complexes of zinc is further demonstrated in Fig. 5, showing distribution of zinc species at different sodium chloride background salt concentrations in ZnCl2 solution (concentration \(m_{{{\text{ZnCl}}_{2}}}\) = 20.0 mmol kg−1water). The speciation plots were generated using PHREEQC, fixing pH at constant values and changing the concentration of background NaCl salt, and keeping ZnCl2 amount constant. At pH 6.0, no precipitation occurred, irrespective of chlorides concentration. An amount of chloro complex ions of zinc increases at NaCl concentrations greater than 0.1 mol kg−1water. In solutions being nearly saturated with NaCl, free Zn2+ cation is absent while complexed [ZnCl4]2– is a major form of zinc in the solution. In the case of the solution having pH 8.0, shown in Fig. 5 (right), zinc exists in precipitated form as ZnO or as Zn5(OH)8Cl2, depending on background NaCl concentration. The composition of precipitates changes from ZnO to Zn5(OH)8Cl2 at NaCl concentration greater than 0.3 mol kg−1water. Further increase of chlorides concentration (above 1.0 mol kg−1water) causes a dissolution of the precipitate. The induction of a zinc precipitate dissolution is used in brine leaching, aimed to selectively dissolve zinc in the presence of iron [31, 32].
Cadmium System
Figure 6 shows the response of chloride complexed cadmium metal to hydroxide precipitation in comparison with uncomplexed metal where only NaOH is used for pH increase. PHREEQC-generated results show that the increase in chlorides concentration to 1300 mmol kg−1water increases an equilibrium solubility of cadmium in the solution by an order of magnitude. Resolubilization of cadmium at higher pH confirms its amphoteric character [33], but the effect occurs at significantly higher pHs, comparing to zinc (Fig. 3). A shift of the pH of minimal Cd solubility is also affected by the chlorides concentration. The addition of 1300 mmol kg−1water of background NaCl to CdCl2 causes shift of the minimum solubility pH from pH 11.8 to 12.2. The shift is much more prominent, compared to zinc system (Fig. 3). In Fig. 6, experimentally observed solubilities of cadmium are included. Bearing in mind that the chemistry of cadmium is very much like that of zinc, except that it is less active and not as acidic in alkaline solution [34], the analogous rationale explaining the variation in the calculated and experimentally observed solubilities can be expressed as in the case of zinc (see the previous chapter).
In the case of cadmium–chloride system, two precipitates compete: Cd(OH)2 and hydroxy salt CdOHCl. Composition of the precipitate is related to the pH and to the concentration of background chlorides. Similarly, as in the case of zinc, the range of pH where CdOHCl is dominant in the precipitate is wider for the solution having a high NaCl concentration (see Fig. 7).
The analysis of Fig. 7 (left) shows that chloride anion strongly complexes cadmium. Even in the absence of additional NaCl background salt, chlorides concentration (Cl– anion in the solution is originated only from CdCl2) is sufficient to complex over 60% of cadmium present. Further decrease in the concentration of free Cd2+ and the increase of concentration of complexes of cadmium are observed with increasing Cl– concentration (Fig. 8).
As with zinc, chloride anion acts as a typical complexing agent inhibiting metal hydroxide precipitation [35] and if present in sufficiently high concentration can even dissolve the precipitate. An example of such a phenomenon is shown in Fig. 8 (right)—cadmium bearing precipitate is being dissolved even at pH 10.0, if the concentration of Cl– exceeds 2 mol kg−1water.
Chloride complexation affects the performance of hydroxide precipitation to a much lesser extent, compared to stronger complexing agents like EDTA, NTA [36], but still increases the solubility of zinc and cadmium, especially in chloride bearing streams like metallurgical wastewaters.
Conclusions
Hydroxide precipitation of zinc, cadmium, and other heavy metals is still the most common and effective method of treatment of heavy metal bearing wastewaters. Although widely used, hydroxide precipitation also has some limitations: the presence of complexing agents in the wastewater, being one of them. The aim of this study was to assess zinc and cadmium solubility in solutions containing a high amount of background chlorides, acting as a complexing agent, in a theoretical and experimental manner. An analysis of such solutions, having extreme ionic strengths exceeding 1.0 mol kg−1water, required the introduction of Pitzer’s model to address electrolyte solution thermodynamics. To generate speciation of solutions and to model precipitation, PHREEQC modeling software was used. The high ionic strength of analyzed solutions affects also a methodology of pH measurements. To avoid errors, connected with using pH combined glass electrodes in highly saline solutions, the method of calibration of pH electrodes was developed and evaluated.
The present analysis showed that both heavy metals zinc and cadmium exhibit similar properties in highly saline solutions. The hydroxide precipitation in such solutions is possible, however, is started to be inhibited by chlorides in concentrations exceeding ~ 0.1 mol kg−1water. Minimum solubility of zinc and cadmium in presence of a high concentration of background salts (i.e., sodium chloride in concentrations > 1.0 mol kg−1water) is decreased by an order of magnitude, compared to solutions lacking background NaCl. The presence of chlorides affects also a composition of the precipitates. The high concentration of chlorides favors the formation of hydroxo salt precipitates Zn5(OH)8Cl2 or CdOHCl instead of typical metal hydroxides.
The relative simplicity and flexibility of the PHREEQC application, created as a geochemical software, proved its value for utilization in wastewater treatment calculations.
References
Blais JF, Djedidi Z, Cheikh RB et al (2008) Metals precipitation from effluents. Pract Period Hazard Toxic Radioact Waste Manage 12:135–149
Bodzek M, Konieczny K, Kwiecińska A (2011) Application of membrane processes in drinking water treatment–state of art. Desalination Water Treat 35:164–184. https://doi.org/10.5004/dwt.2011.2435
Wang LK, Vaccari DA, Li Y, Shammas NK (2005) Chemical precipitation. Physicochemical treatment processes. Humana Press, Totowa, pp 141–197
Charerntanyarak L (1999) Heavy metals removal by chemical coagulation and precipitation. Water Sci Technol 39:135–138
Li H, Chen Y, Long J et al (2017) Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. J Hazard Mater 333:179–185. https://doi.org/10.1016/j.jhazmat.2017.03.020
Ye M, Li G, Yan P et al (2017) Production of lead concentrate from bioleached residue tailings by brine leaching followed by sulfide precipitation. Sep Purif Technol 183:366–372. https://doi.org/10.1016/j.seppur.2017.04.020
Lin X, Burns RC, Lawrance GA (2005) Heavy metals in wastewater: The effect of electrolyte composition on the precipitation of cadmium (II) using lime and magnesia. Water Air Soil Pollut 165:131–152
Hahne HCH, Kroontje W (1973) Significance of pH and chloride concentration on behavior of heavy metal pollutants: mercury(II), cadmium(II), zinc(II), and lead(II). J Environ Qual 2:444–450
Wilson GS, Buck RP, Rondinini S et al (2002) Measurement of pH. Definition, standards, and procedures. CRC Press, Boca Raton
Hyvärinen O, Hämäläinen M (2005) HydroCopperTM: a new technology producing copper directly from concentrate. Hydrometallurgy 77:61–65. https://doi.org/10.1016/j.hydromet.2004.09.011
Haavanlammi K (2014) Nickel matte chloride leaching. Outotec SEAP Customer eNewsletter 3
Marvin E (2013) pH Measurement in high ionic strength brines: calibration of a combined glass electrode to obtain accurate pH measurements for use in a coupled single pass SWRO boron removal model
Spitzer P, Fisicaro P, Meinrath G, Stoica D (2011) pH buffer assessment and Pitzer’s equations. Accred Qual Assur 16:191–198. https://doi.org/10.1007/s00769-010-0743-0
ISO 10523:2008 (2008) Water quality—determination of pH
Parkhurst DL (1995) User’s guide to PHREEQC: a computer program for speciation, reaction-path, advective-transport, and inverse geochemical calculations. US Geological Survey, Denver
Brönsted JN (1922) Studies on solubility. IV. The principle of the specific interaction of ions. J Am Chem Soc 44:877–898. https://doi.org/10.1021/ja01426a001
Truesdell AH, Jones BF (1974) WATEQ, a computer program for calculating chemical equilibria of natural waters. J Res US Geol Surv 2:233–248
Pitzer KS (1973) Thermodynamics of electrolytes. I. Theoretical basis and general equations. J Phys Chem 77:268–277. https://doi.org/10.1021/j100621a026
Plummer LN, Parkhurst DL, Fleming GW, Dunkle SA (1988) A computer program incorporating Pitzer’s equations for calculation of geochemical reactions in brines. U.S. Geological Survey, Reston
Kim HT, Frederick WJ Jr (1988) Evaluation of Pitzer ion interaction parameters of aqueous electrolytes at 25 °C. 1. Single salt parameters. J Chem Eng Data 33:177–184
Rard JA, Miller DG (1989) Isopiestic determination of the osmotic and activity coefficients of ZnCl2(aq) at 298.15 K. J Chem Thermodyn 21:463–482. https://doi.org/10.1016/0021-9614(89)90164-X
Wang D, Yang Y-Y, Zhang X-P, Sang S-H (2016) Mean activity coefficients of NaCl in NaCl–CdCl2–H2O ternary system at 298.15 K by potential difference method. J Chem Eng Data 61:3027–3033. https://doi.org/10.1021/acs.jced.6b00075
Anstiss RG, Pitzer KS (1991) Thermodynamics of very concentrated aqueous electrolytes: LiCl, ZnCl2, and ZnCl2–NaCl at 25 °C. J Solut Chem 20:849–858. https://doi.org/10.1007/BF01074948
Reardon EJ, Armstrong DK (1987) Celestite (SrSO4(s)) solubility in water, seawater and NaCl solution. Geochim Cosmochim Acta 51:63–72. https://doi.org/10.1016/0016-7037(87)90007-X
Baltpurvins KA, Burns RC, Lawrance GA, Stuart AD (1997) Effect of electrolyte composition on zinc hydroxide precipitation by lime. Water Res 31:973–980
Cousy S, Gorodylova N, Svoboda L, Zelenka J (2017) Influence of synthesis conditions over simonkolleite/ZnO precipitation. Chem Pap 71:2325–2334. https://doi.org/10.1007/s11696-017-0226-4
Takada T, Kiyama M, Torii H, et al (1978) Effect of pH values on the formation and solubility of zinc compounds
Cousy S, Svoboda L, Zelenka J (2013) Basic precipitation of simonkolleite nanoplatelets. In: Proceedings of 5th International Conference NANOCON 2013, pp 16–18
Patterson JW, Petropoulou C, Luo B et al (1991) Differential precipitation applications for metals separation and recovery. In: Türkman A, Uslu O (eds) New developments in industrial wastewater treatment. Springer, Dordrecht, pp 201–212
Robinson RA, Stokes RH (1959) Electrolyte solutions, 2nd edn. Dover Publications, Mineola
Barrera-Godinez JA, O’Keefe TJ, Watson JL (1992) Effect of ultrasound on acidified brine leaching of double-kiln treated EAF dust. Miner Eng 5:1365–1373. https://doi.org/10.1016/0892-6875(92)90172-6
Farahmand F, Moradkhani D, Safarzadeh MS, Rashchi F (2009) Brine leaching of lead-bearing zinc plant residues: process optimization using orthogonal array design methodology. Hydrometallurgy 95:316–324. https://doi.org/10.1016/j.hydromet.2008.07.012
Dirkse TP (1986) Cadmium oxide and hydroxide. In: Copper, silver, gold and zinc, cadmium, mercury oxides and hydroxides. International union of pure and applied chemistry
Mellor JW (1946) A comprehensive treatise on inorganic and theoretical chemistry. Longmans, Green, London
Purkayastha D, Mishra U, Biswas S (2014) A comprehensive review on Cd(II) removal from aqueous solution. J Water Process Eng 2:105–128. https://doi.org/10.1016/j.jwpe.2014.05.009
Tünay O, Kabdaşli NI (1994) Hydroxide precipitation of complexed metals. Water Res 28:2117–2124. https://doi.org/10.1016/0043-1354(94)90022-1
Acknowledgements
This work was supported by the “Effective generation of electric power from metallurgical waste gases with a simultaneous reduction of chlorine ion emissions into the environment”—RECLEG project co-financed by the European Regional Development Fund under the Operational Programme Smart Growth (Contract No.: POIR.01.02.00-00-0170/16) within Sectoral Programme INNOSTAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was T. Hirato.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stec, M., Jagustyn, B., Słowik, K. et al. Influence of High Chloride Concentration on pH Control in Hydroxide Precipitation of Heavy Metals. J. Sustain. Metall. 6, 239–249 (2020). https://doi.org/10.1007/s40831-020-00270-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00270-x












