Abstract
The harmful trace elements will be released during coal utilization, which can cause environment pollution and further endangering human health, especially for heavy metal elements. Compared to combustion, the release of heavy metal elements during coal pyrolysis process, as a critical initial reaction stage of combustion, has not received sufficient attention. In the present paper, a low rank coal, from Xinjiang province in China, was pyrolyzed in a fixed bed reactor from room temperature, at atmospheric pressure, with the heating rate of 10 °C/min, and the final pyrolysis temperature was from 400 to 800 °C with the interval of 100 °C. The volatility of heavy metal elements (including As, Hg, Cd and Pb) during pyrolysis process was investigated. The results showed the volatility of all heavy metal elements increased obviously with increasing temperature, and followed the sequence as Hg > Cd > As > Pb, which was mainly caused by their thermodynamic property and occurrence modes in coal. The occurrence modes of heavy metals were studied by sink-and-float test and sequential chemical extraction procedure, and it can be found that the heavy metal elements were mainly in the organic and residual states (clay minerals) in the raw coal. And most of the organic heavy metals escaped during the pyrolysis process, the remaining elements were mainly in the residual state, and the elements in Fe–Mn state also tended to remain in the char.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
China is the largest producer and consumer of coal all over the world, accounting for about 37% of world production of coal (Zhou et al. 2016). Coal utilization will not only discharge SO2, nitrogen oxides (NOx), CO2 and other air pollutants, the harmful trace elements containing in coal and their compounds will also be released, which bring pollution to water, soil and other ecological environment further endangering human health. The release behavior of heavy metal elements during coal combustion has attracted wide attention both at home and abroad. In the 1990s, some trace elements, including Hg, As, Cd and Pb (Zhou et al. 2019a, b), have been listed on the U.S. Clean Air Act as key toxic air pollutants. The European Union, Japan and other developed countries also have a clear emissions indicator of these heavy metal elements for the coal-fired power plants. Many scholars have conducted researches on the release and transfer behaviors of harmful trace elements in coal combustion process. However, pyrolysis as a critical initial reaction phase of combustion (Gürüz et al. 2004), the release of heavy metal elements in the process has not received sufficient attention.
The release behavior of heavy metal elements during coal combustion process has been extensively investigated (Zhang et al. 2003a, b; Qin 2005; Zhao 2008; Querol et al. 1995; Smith 1980; Frandsen et al. 1994; Miller et al. 2003). According to literature, the release behavior of heavy metal elements during coal combustion was not only dependent on their thermodynamic property (i.e. Hg owned low boiling point, the volatility of Hg will be higher than other elements.), but also on their occurrence modes (Wu et al. 2013). The heavy metal elements present in the coal can be divided into organic bound states (organic, ionic or water-soluble) and mineral-bound states (including primary and secondary minerals) (Davidson and Clarke 1996). It was shown that heavy metal elements bound in the organic state were more volatile than the mineral-bound elements in the combustion process (Linak and Wendt 1993), whereas the elements present in the mineral-bound state were more affected by the mineral species. Duan et al. (2017) found that pyrite-related elements always exhibited high volatility, whereas Sekine et al. (2008) reported that heavy metal elements in clay minerals (residual form) were usually released slowly with increasing temperature.
However, few literature studied the release behavior or volatility characteristics of heavy metal elements during coal pyrolysis process (Wang et al. 2002; Zajusz-Zubek and Konieczynski 2003; Guo et al. 2003, 2004a, b), which were not enough to reveal its release mechanism. Some researchers (Wang et al. 2002; Zajusz-Zubek and Konieczynski 2003) reported the distribution of heavy metal elements in the pyrolysis products and compared the volatility of several heavy metal elements such as As, Pb, Cd and Hg, without the explanation for the distribution behavior. Guo et al. (2003, 2004a, b) did lots of work and found that the volatility of heavy metals increased with the increasing pyrolysis temperature and residence time, decreased at advanced pressure, the hydrogen atmosphere was favorable for the evaporation of heavy metal elements, and the occurence mode of heavy metals was studied by the indirect method, named sequential chemical extraction. It can be found that the occurrence mode of elements might be a crucial factor for the volatility of heavy metals during pyrolysis or combustion process. Therefore, it is necessary to investigate the occurrence mode of elements in coal, besides the influence of pyrolysis process conditions. In addition, among so much literature, rare report studied the volatility of heavy metals during thermal-processing for low rank coal, normally owned low sulfur content. Low rank coal owns high volatile content and reactivity, which make it a good raw material to coal chemistry. It is necessary to study the release behavior of heavy metal elements during pyrolysis process for low rank coal, for the environmental issue.
In the present study, a low rank coal from Xinjiang province in China, named MZH coal, was pyrolyzed in a fixed bed reactor from room temperature, at atmospheric pressure, with the heating rate of 10 °C/min, and the final pyrolysis temperature was from 400 to 800 °C with the interval of 100 °C. The release behavior of heavy metal elements (including As, Hg, Cd and Pb) during pyrolysis process was investigated. Except for the indirect sequential chemical extraction method, the occurrence modes in MZH coal were also studied by a direct method, sink-and-float test, by analysis the distribution of heavy metal elements and the mineral matters in different density fractions of MZH coal.
2 Materials and methods
2.1 Materials
The sample used was a Chinese low rank coal from Xinjiang province, named MZH coal, a low-rank bituminous coal owned very high volatile matter. The raw materials were crushed and milled to get the particle size less than 2 mm. All samples were heated to 105 °C and maintained at this temperature for 24 h in an air-dried oven to get rid of the moisture. The characteristics of raw coal were performed according to the related standards. The ultimate and proximate of raw sample are shown in Table 1. It can be found that MZH bituminous coal owed very high volatile matter, around 50 wt%, showing its low-rank. The low ash (9.95 wt%) and sulfur (0.50 wt%) content showed that the content of these trace elements would be also low, since most heavy metals such as As, Hg, and Cd were reported to be mainly associated with sulfide minerals (Duan et al. 2017).
2.2 Methods
2.2.1 Pyrolysis experiments in a fixed bed reactor
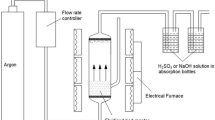
Pyrolysis experiments of MZH coal were conducted in a fixed-bed reactor as schematically illustrated in Fig. 1 (Chang et al. 2017). The reactor was made of stainless steel, and a stainless-steel pipe was welded to the cover of the reactor for carrier gas inlet. Approximately 50 g of raw MZH coal was placed inside the reactor, which was installed in an electric-ring furnace with a thermocouple inserted. Then, it was heated from ambient temperature to final temperature of 400–800 °C with the interval of 100 °C, at a heating rate of 10 °C/min and held at the final temperature for 60 min. Nitrogen gas was introduced into the reactor at a flow rate of 50 mL/min to sweep the volatile products out of the reactor. The evolved oil vapor, water steam, and gases were channeled into a conical flask that was immersed in an ice-water bath. Most of the water and oil condensed in the flask, and the residual portion was captured by a drying tube filled with cotton wool and silica gel. The non-condensable gases were collected in gas bags, which was changed every 100 °C intervals to investigate the gaseous products with increasing temperature. After the conclusion of each run, the apparatus was disassembled. The mass of MZH char was weighed directly, and the mass of the mixture of tar oil and water was determined from the mass difference between the collecting flask before and after pyrolysis. The water in the oil/water mixture was determined by the Dean-Stark method using toluene as a solvent.
2.2.2 Pyrolysis experiments in thermogravimetric analyzer
To investigate the release behavior of heavy metals during pyrolysis process, pyrolysis behaviors of MZH coal (10 mg) in TGA at 10 °C/min from room to 900 °C and atmospheric pressure are studied, by utilizing mass loss (TG) and reaction rate (DTG) plots. The thermogravimetric analysis (TGA) was carried out using a Mettler-Toledo TGA/DSC 1 thermogravimetric analyzer. Nitrogen was used as the carrier gas (at 50 mL/min) in order to ensure an oxygen-free environment.
2.2.3 Sink-and-float test
Coal with different densities contains different amounts and types of minerals. The major constituents of the mineral matter in coal include quartz (2.65 g/cm3), pyrite (5.00 g/cm3), calcite (2.71 g/cm3) and clay (2.90 g/cm3), compared to much less density for organic matter (< 1.4 g/cm3) (Luo et al. 2011). The sink-and-float separation was done as follows: The heavy fluids were allocated at 1.25, 1.30, 1.35, 1.40 and 1.45 g/cm3, respectively, then put into five buckets and calibrated by a densitometer. Around 987.84 g MZH coal sample in a sieve basket, with particle size of 0.5–2 cm, was firstly put into the heavy fluids with lowest density. When stratification of coal particles finished, the float fraction was separated by a small spoon, which cannot exceed the depth of 10 mm in the bucket. The sink fraction will stay in the sieve basket, then was slowly lift from the lowest heavy liquid. This sink fraction would be put in the next bucket with higher density, and the float and sink fractions were obtained by the same way one after the other in sequence until all the samples have been tested. By sink-and-float test, the MZH coal was separated into six density fractions of < 1.25, 1.25–1.30, 1.30–1.35, 1.35–1.40, 1.40–1.45 and > 1.45 g/cm3. The densities in the present study were much lower, due to the low ash content for MZH coal. Then, the fractions with difference densities were analyzed to determine the ash composition by X-ray diffraction (XRD), and to measure the contents of heavy metals by measurement in Table 3. The ashes for all samples were produced at low temperature of 250 °C for several days to avoid the crystal transition. By sink-and float test, the relationship between mineral matters and occurrence mode of heavy metals can be roughly obtained.
2.2.4 Sequential chemical extraction procedure
The heavy metals (As, Cd and Pb) in the raw coal and chars were separated into five chemical forms (heavy metals as ion exchangeable, bound to carbonates, bound to Fe–Mn oxide, bound to organic matter and remained in the residue) by a sequential chemical extraction procedure. Through the procedure, heavy metals associated with different parts of the coal can be removed in the following order shown in Table 2. Then, the extracts were measured by inductively coupled plasma atomic spectroscopy (ICP-AES) to investigate the occurrence mode.
2.2.5 Measurement of heavy metal elements
The measurement methods of the heavy metal elements were shown in Table 3. The mercury analyzer was used to measure the Hg content, which theoretically was a Cold atomic absorption spectrometry. The content of As was analyzed by Arseno-antimono-molybdenum blue spectrophotometry, which was more suitable for As compared to the ICP (inductively coupled plasma). The content of Cd and Pb were measured by ICP-MS (inductively coupled plasma-mass spectrometer) to ensure the accuracy of the data.
2.2.6 The volatility of the heavy metal elements
The volatility of heavy metal elements is used to evaluate the extent of the elements released during coal pyrolysis and is defined as:
where R(x) is the volatility of the elements; x can be element Hg, As, Cd and Pb; Coal(x) is the weight of x in raw coal; Char(x) is the weight of x in char. The weight of element x in coal or char can be obtained by the content of element x measured multiply by the weight of raw coal or char.
3 Results and discussion
3.1 Occurrence modes of heavy metals
3.1.1 Occurrence modes of heavy metals by sink-and-float test
1. Characteristics of coal in different densities by sink-and-float test
By sink-and-float test, the MZH coal was separated into six density fractions of < 1.25, 1.25–1.30, 1.30–1.35, 1.35–1.40, 1.40–1.45 and > 1.45 g/cm3. These products were washed with hot water to get rid of the heavy fluid (zinc chloride), until the water was not significantly white. Then, the wet products was dried by a electricity drying oven. The dry products were weight and analyzed, and the basic properties, including weight, yield, proximate analysis and ultimate analysis, were shown in Table 1. It can be seen that the density of raw MZH coal was low, mainly in the range of 1.30–1.45 g/cm3. For each density product, the ash content increased from 4.46 wt% to 11.95 wt% with the increasing densities. The content of total sulfur decreased from 0.30 wt% to 0.23 wt% firstly, then increased from 0.23% to 0.32 wt%. The ash and sulfur content were closely related with the occurrence modes of heavy metal elements, which will be discussed later.
2. Ash composition of coal in different densities by sink-and-float test
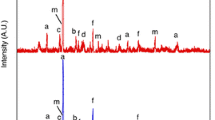
The ash composition for MZH coals with different densities was measured by XRD and shown in Fig. 2. By comparison, it can be found that the clay minerals (K: Kaolinite, F: Feldspar, M: Mica) increased in larger-density coal. The content of sulfate minerals also increased with increasing densities, which transformed by carbonate and sulfur in raw coal. In addition, there were many peaks for zinc oxide, since the heavy liquid (zinc chloride) was difficult to be washed away completely.
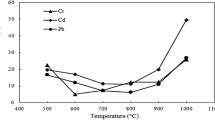
3. Heavy metal elements of coal in different densities by sink-and-float test
The content of heavy metal elements contained in coals with different densities are shown in Fig. 3. It can be seen that as the increasing density, the trend of these elements was as follows:
-
(1)
With the increasing coal density, the content of Hg decreased firstly from 0.018 (< 1.25 g/cm3) to 0.01 μg/g (1.30–1.35 g/cm3), increased slowly to 0.011 μg/g (1.40–1.45 g/cm3), then sharply went to 0.024 μg/g for coal with highest density. The content of As followed similar trend as that of Hg. According to literature (Luo et al. 2011), a large proportion of Hg and As in coal was associated with pyrite. Table 1 shows that the content of total sulfur followed the similar trend, which decreased firstly then increased quickly with higher density. In addition, the determined iron content increased with increasing density, indicating the high content of pyrite in high-density coal. For low rank coal, As can be partly present in the organic matter, and Hg could be associated with selenides, galena and carbonates.
-
(2)
With the increasing coal density, the content of Cd stayed constantly at 1.0 μg/g until the density went to 1.45 g/cm3, then increased sharply to 2.0 μg/g for coal with highest density. According to the ash composition of different-density coals in Fig. 2, it can be deduced that a part of Cd existed in the form of carbonate and clay minerals, which emerged at XRD patterns for highest-density coal. It was reported that there was also a small amount of Cd presented in the pyrite and organic matter in coal.
-
(3)
With the increasing coal density, Pb content increased obviously from 55 to 230 μg/g. The occurrence modes of Pb were diverse, in which the proportion of sulfide bound state was slightly higher, and the content of silicon-aluminum bound state, carbonate bound state, organic state to ion exchangeable and water soluble state were sequentially decreased. Pb was mainly found in galena, selenium ore or associated with other sulfides. It can be seen from XRD that the content of carbonate and clay minerals increased, resulting in a significant increase in Pb content.
-
(4)
To sum up, by analysis of ash composition and heavy metal elements distribution for coal with different densities obtained by sink-and-float test, it can be deduced that the main occurrence modes of heavy metals in MZH coal, especially for the elements exited as inorganic form, were shown as follows:
-
① Mercury Hg: mainly associated with pyrite;
-
② Arsenic As: mainly associated with pyrite;
-
③ Cadmium Cd: mainly accompanied by clay minerals and pyrite;
-
④ Lead Pb: mainly accompanied by carbonate, clay minerals and sulfide.
-
3.1.2 Occurrence modes of heavy metals by sequential chemical extraction procedure
Figure 4 illustrates distribution of the heavy metal elements in five chemical form (heavy metals as ion exchangeable, bound to carbonates, bound to Fe–Mn oxide, bound to organic matter and remained in the residue) in the coal and the corresponding chars obtained from pyrolysis at 800 °C and a holding time of 1 h. For raw MZH coal, it is interesting to found that the distribution of As, Pb and Cd in the coal were similar, which large portions of the elements remained in the residue fraction and bound to organic matter after the preceding extractions and the rest part mainly bound to Fe–Mn oxide. The elements associated with carbonates and as ion exchangeable state accounted for very small part. However, the dominant forms of heavy metals in coals mentioned here were reported to be associated with sulfides in most literature, such as pyrite, galena, or sphalerite, while hardly any literature mentioned a large proportion of heavy metals associated with organic matters. Zhou et al. (2019a, b) studied the effects of occurrence mode of Cd on its volatility during pyrolysis process for two low rank coals, and found the similar phenomenon, which the main form for Cd in coals were bound to organic matter and residue fraction. It can be speculated that the association with organic matter is a feature of low rank coal, since low rank coal contains more heteroatoms (such as sulfur and nitrogen). Transition metals (including the heavy metal elements mentioned in the present study) could readily form complexes with heteroatoms in low rank coals due to the unfilled valence d orbitals (William 2003).
By combination of sink-and-float test and sequential chemical extraction procedure, the occurence modes of heavy metal elements in MZH can be summarized as follows:
-
(1)
Mercury Hg: pyrite + organic state (deduced by pyrolysis behavior);
-
(2)
Arsenic As: pyrite + organic state (main form) + residue state (clay minerals);
-
(3)
Cadmium Cd: pyrite + organic state + residue state (clay minerals);
-
(4)
Lead Pb: pyrite + carbonate + organic state (main form) + residue state (clay minerals).
3.2 Pyrolysis behavior of raw coal
3.2.1 Pyrolysis behaviors in TGA
As shown in Fig. 5a for TG plot of MZH coal, the first stage was from the beginning to around 360 °C, where only water and a few peripheral mobile phase from the macromolecular structure decomposed. The second stage was from 360 to about 500 °C, where big weight loss, approximately 22 wt%, was produced. The maximum decomposition peak showed at around 440 °C, corresponding to the sharp decrease in TG plot, caused by big amount volatiles produced in a narrow temperature range. As a low rank coal, there were higher number of oxygen-containing functional groups contained in MZH coal, which could decompose quickly at moderate temperature. This can cause the release of heavy metals bound to organic matter. The third stage was from 500 to 900 °C, where the remaining macromolecular structures of coal, mainly dense polycyclic aromatic compounds in the immobile phase, decomposed further at a relatively lower rate producing about 25 wt% volatile. At high temperature, some minerals would decompose or transform, leading to the release of heavy metals associated with these minerals. The total wight loss in TGA was very high, which was more than 60 wt%.
3.2.2 Products yields during pyrolysis in fixed bed reactor
To investigate the migration behavior of heavy metal elements during pyrolysis in fixed bed reactor, it is necessary to determine the distribution of heavy metal elements in the pyrolysis products. The products (including char, tar and chemical bond water) yields during pyrolysis process are shown in Fig. 6. It can be found that products yields of coke, tar and chemical water showed different trends with increasing temperature from 400 to 800 °C. The char yield gradually reduced from 70 wt% at 400 °C to 51 wt% at 800 °C. With increasing pyrolysis temperature, coal continuously generated CO2, CO, CH4, H2 and unsaturated hydrocarbons, leading to the reduction of char yield. The yields of chemical water and tar increased a little between 400 and 500 °C. At higher temperatures, the yields of chemical water kept constant, while the tar yield decreased slightly with increasing temperature. The tar products were mainly formed in the primary pyrolysis stage (300–600 °C). At higher temperature range, tar products would precipitate the secondary pyrolysis reaction with gaseous products or char to produce secondary char, leading to reduction of tar yield.
3.2.3 Gas composition during pyrolysis in fixed bed reactor
The gas generated during the pyrolysis process had big effect on the release and migration behavior of heavy metal elements in the coal. Therefore, the non-condensable gases were collected in gas bags every 10 min (100 °C) to investigate the gaseous products with increasing temperature, and the gases were collected from 300 °C (0 min). The relationship between gas composition and residence time is shown in Fig. 7. It can be found that CO2 was firstly produced during coal pyrolysis process, then CO and CH4 was released at higher temperature, and finally lots of H2 was produced. For Fig. 5a, corresponding to final temperature at 400 °C, the content of CO2 was highest, since carboxylic compounds were the most thermally unstable functional groups among the oxygen-containing functional groups in the chemical structures, which was easy to be decomposed at low temperature. When temperature was higher than 500 °C, a lot of H2 was produced, which the ordering of gas content was H2 > CH4 > CO ≥ CO2. In addition, it can be seen that at the final temperature, most gas yield reached its peak when the constant time was 20–40 min, showing that 1 h keeping at the final temperature was enough to measure the pyrolysis behaviors.
3.3 The volatility of heavy metals during pyrolysis in fixed bed reactor
3.3.1 The characteristics of MZH chars pyrolyzed at different temperatures
To investigate the volatility of heavy metal elements during pyrolysis of MZH coal, the char characteristics for raw coal and char samples at different final pyrolysis temperature were shown in Table 4. It can be found that the ash content for chars increased obviously from 9.95 wt% for raw coal to 17.67 wt% for char pyrolyzed at 800 °C. In addition, the sulfur content which had big relationship with the content of heavy metal elements, decreased firstly from 0.50 wt% for raw MZH coal to 0.45 wt% for char pyrolyzed at 500 °C, then increased to 0.54 wt% for char pyrolyzed at 800 °C. The oxygen content dropped sharply from 10.81 wt% to near to zero at higher temperature, showing mostly oxygen-containing groups consumed during pyrolysis process. All of these changes in the properties of the char will have important effects on the release of heavy metal elements.
3.3.2 The release behavior of heavy metals during pyrolysis process
To obtain the volatility of heavy metal elements during pyrolysis of MZH coal, the contents of these elements in char samples were firstly measured. Then the absolute weight of these elements in raw and char samples were calculated based on the content and the weight of MZH and char samples. Finally, the volatility R(x) of heavy metal elements can be obtained by Eq. (1). The volatility of heavy metal elements including Hg, As, Cd and Pb during pyrolysis of MZH coal was shown in Fig. 8.
It can be found that the volatility of all heavy metal elements increased obviously with increasing temperature, and followed the sequence as Hg > Cd > As > Pb, which was consistent with the findings in literature (Wang et al. 2002). The release or migration behaviors of heavy metal element depended on its mode of occurrence in coal and the pyrolysis behaviors. Therefore, the release behavior and its explanation for each heavy metal element was illustrated as follows.
-
(1)
The release behavior of Hg (Mercury)
According to literature, the inorganic mercury in coal was mainly existed in the form of Hg elementary substance, chloride, HgS and so on (Zhang et al. 2003a, b). The boiling point of these inorganic mercury was very low, mostly below 400 °C (Zhang et al. 2003a, b), so around 50 wt% Hg released at 400 °C. For organic form, Hg could react with H2, HCl and H2S in the volatile products. As can be seen from Fig. 7, lots of H2 produced when temperature higher than 500 °C, so Hg escaped constantly as the temperature increases (> 90 wt%).
-
(2)
The release behavior of As (Arsenic)
Organic As can be combined with oxygen and sulfur in coal macromolecules, which can be released as the migration of oxygen and sulfur during pyrolysis process. Therefore, around 30% As escaped at low temperatures (400 °C) indicated that this part of As was present in organic form in coal. For inorganic arsenic, As2S3 is the main substance in most coal, but the boiling point of As2S3 is higher than 800 °C. As2S3 can react with H2 (produced at high temperature) and released as AsH3 (Liu 2011). However, the content of sulfur in MZH coal was very low (St, 0.50 wt%), so the volatility of As at higher temperature was much lower than that of Hg (45 wt% compared to 93 wt%).
-
(3)
The release behavior of Cd (Cadmium)
The organic Cd element can be decomposed and evaporated before 800 °C. Therefore, around 37 wt% Cd escaped at low temperatures of 400 °C indicate that this part of Cd was present in organic form. For inorganic cadmium, the sink-float experiment showed that the inorganic Cd in MZH coal was mainly present with clay minerals and sulfides, which was hard to release (boiling point was greater than 1000 °C). Cd existed in carbonates and oxide can be reduced by char before 800 °C. Therefore, the volatility of Cd increased with increasing temperature (around 75 wt%).
-
(4)
The release behavior of Pb (Lead)
Organic-bound Pb can be decomposed and volatilized before 500 °C, so the volatilization rate at 500 °C was about 17%. For inorganic lead, the Pb oxide and the sulfide of lead can be directly reacted with hydrogen chloride (the chlorine was released as HCl at low temperature and volatilized in the form of alkali metal such as KCl at high temperature) or chlorine gas to form lead chloride (Wu et al. 2013). Therefore, by generation of chlorine, the volatility of Pb enhanced with increasing temperature. In addition, the aluminosilicate in coal could react with volatile Pb to inhibit its release, resulting in a decrease at 600 °C. Pb associated with minerals such as aluminosilicates and sulfides had high thermal stability and were generally difficult to volatilize. But at around 800 °C, sulfides can reacted with CaO (from carbonate decomposition) and char, resulting in Pb release at high temperatures.
3.3.3 Release mechanism of heavy metal elements
The release mechanism and occurrence modes of heavy metals during pyrolysis were summarized and deduced in Table 5. It can be seen that the volatility of heavy metal elements in order was Hg (93 wt%) > Cd (75 wt%) > As (45 wt%) > Pb (25 wt%), which was determined by the characteristics of elements and their occurrence modes in coal. It can be seen from Table 5 that the release of heavy metal elements in the organic state accounted for a large part, namely Hg (43 wt%), As (30 wt%), Cd (37 wt%) and Pb (17 wt%), which was consistent with their occurrence modes. By sequential chemical extraction procedure shown in Fig. 4a, the heavy metals (Hg, As, Pb and Cd) for raw MZH coal were mainly in the organic states and residual states. As shown in Fig. 4b, by pyrolysis at 800 °C, most of the organic heavy metals escaped, the remaining elements were mainly from the residual state, and the elements in Fe–Mn state also tended to remain in the char. This was consistent with the experiment behaviors described previously. The residual state was the elements mainly present in the clay minerals, which was difficult to be volatilized before 800 °C. Therefore, combined with the occurrence modes and the release behaviors of heavy metals in organic and inorganic states, the migration mechanism of each heavy metal element can be derived.
4 Conclusion
The present study mainly focused on effects of occurrence modes of heavy metal elements on the release behaviors during pyrolysis process. From the discussions, it can be concluded that:
-
(1)
By combination of sink-and-float test and sequential chemical extraction procedure, the occurence modes of heavy metal elements in MZH can be summarized. Hg was bound to pyrite and organic state (deduced by pyrolysis behavior); As was associated with pyrite, organic state (main form) and residue state; Cd was bound to pyrite, organic state and residue state; Pb was associated with pyrite, carbonate, organic state (main form) and residue state. The association with organic matter was a feature of low rank coal, since low rank coal contains more heteroatoms, which could form complexes with heavy metal elements.
-
(2)
For the release behaviors of heavy metal elements, the volatility of all heavy metals increased obviously with increasing temperature, and followed the sequence as Hg (93 wt%) > Cd (75 wt%) > As (45 wt%) > Pb (25 wt%), which was mainly caused by their different occurrence modes. By pyrolysis, most of the organic-bound heavy metals escaped, the remaining elements were mainly as the residual state, and the element in Fe–Mn state tended to remain in the char.
References
Chang Z, Chu M, Zhang C et al (2017) Investigation of the effect of selected transition metal salts on the pyrolysis of Huadian oil shale, China. Oil Shale 34(4):354–367
Davidson RM, Clarke LB (1996) Trace elements in coal. Fuel Energy Abstr 37(3):230
Duan P, Wang W, Liu X et al (2017) Distribution of As, Hg and other trace elements in different size and density fractions of the Reshuihe high-sulfur coal, Yunnan Province, China. Int J Coal Geol 173:129–141
Frandsen F, Dam-Johansen K, Rasmussen P (1994) Trace elements from combustion and gasification of coal—an equilibrium approach. Prog Energy Combust Sci 20(2):115–138
Guo R, Yang J, Liu D et al (2003) Influence of thermal treatment conditions on transformation of trace elements in Datong coal. CIESC J 54(11):1603–1607
Guo R, Yang J, Liu Z (2004a) Behavior of trace elements during pyrolysis of coal in a simulated drop-tube reactor. Fuel 83(6):639–643
Guo R, Yang J, Liu Z (2004b) Volatility of trace harmful elements in coal. China Environ Sci 24(6):641–645
Gürüz GA, Ünalp Ü, Durusoy T (2004) Mathematical modeling of thermal decomposition of coal. J Anal Appl Pyrolysis 71(2):537–551
Linak WP, Wendt JOL (1993) Toxic metal emissions from incineration: mechanisms and control. Prog Energy Combust Sci 19(2):145–185
Liu Y (2011) Study on migration mechanism of harmful elements in coal pyrolysis porocess. Sci Technol Inf 27(465):839
Luo G, Yao H, Xu M et al (2011) Identifying modes of occurrence of mercury in coal by temperature programmed pyrolysis. Proc Combust Inst 33:2763–2769
Miller B, Dugwell DR, Kandiyoti R (2003) The influence of injected HCl and SO2 on the behavior of trace elements during wood-bark combustion. Energy Fuels 17(5):1382–1391
Qin P (2005) Study on the rules of heavy metals produced by coal combustion. Master Thesis. Zhejiang University
Querol X, Fernández-Turiel JL, Lopez-Soler A (1995) Trace elements in coal and their behaviour during combustion in a large power station. Fuel 74(3):331–343
Sekine Y, Sakajin K, Kikuchi E et al (2008) Release behavior of trace elements from coal during high-temperature processing. Powder Technol 180(1):210–215
Smith RD (1980) The trace element chemistry of coal during combustion and the emissions from coal-fired plants. Prog Energy Combust Sci 6(1):53–119
Wang Y, Li H, Huang H et al (2002) Distribution and transport of heavy metal elements during coal pyrolysis. Coal Convers 25(3):37–42
William JB (2003) The place of zinc, cadmium, and mercury in the periodic table. J Chem Educ 80(8):952
Wu H, Glarborg P, Frandsen FJ et al (2013) Trace elements in co-combustion of solid recovered fuel and coal. Fuel Process Technol 105(1):212–221
Zajusz-Zubek E, Konieczyński J (2003) Dynamics of trace elements release in a coal pyrolysis process. Fuel 82(10):1281–1290
Zhang J, Zheng C, Liu J et al (2003a) Experimental study on volatility of trace metals in coal combustion. J Eng Thermophys 24(6):1043–1046
Zhang J, Han CL, Xu YQ (2003b) The release of the hazardous elements from coal in the initial stage of combustion process. Fuel Process Technol 84(1–3):121–133
Zhao Y (2008) Partition mechanism and interaction of minerals and trace elements during coal combustion. PhD thesis. Huazhong University of Science and Technology
Zhou LM, Zhang GJ, Schurz M et al (2016) Kinetic study on CO2 gasification of brown coal and biomass chars: reaction order. Fuel 173:311–319
Zhou L, Guo H, Chu M et al (2019a) Release mechanism of Cd in low rank coal during pyrolysis process. J China Coal Soc 44(1):323–331
Zhou L, Wang X, Guo H et al (2019b) Research progress on influence of heavy metal occurrence mode on release behavior during coal conversion. Clean Coal Technol 24(6):8–13
Acknowledgements
The authors are grateful to the financial support of the National Key Research and Development Program of China (2016YFB0600304) and the National Natural Science Foundation of China (No. 51804313).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhou, L., Guo, H., Wang, X. et al. Effect of occurrence mode of heavy metal elements in a low rank coal on volatility during pyrolysis. Int J Coal Sci Technol 6, 235–246 (2019). https://doi.org/10.1007/s40789-019-0251-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-019-0251-8