Abstract
Background
Sarcopenia has been associated with increased systemic inflammation and risk of physical disability in older adults. Recently, extracellular heat shock protein 72 (eHSP72) was proposed as a biomarker of sarcopenia but its response to interventions designed to increase muscle mass has never been evaluated.
Aims
The present study was designed to (1) assess eHSP72 levels following resistance training and, (2) determine whether changes in eHSP72 correlate to changes in muscle mass and inflammatory markers.
Methods
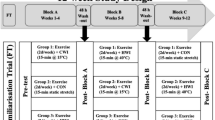
A total of 26 sarcopenic men participated in a 16-week resistance training program. The following variables were measured pre–post-intervention: plasma HSP72, serum high sensitivity (hs) inflammatory markers: interleukin-6 (hsIL-6), C-reactive protein (hsCRP), and tumor necrosis factor alpha (hsTNF-α), lean body mass (LBM) and appendicular muscle mass index (appMMI).
Results
eHSP72 was detected in 47 % of our participants and its level significantly decreased (P = 0.04) after the intervention, with a concomitant increase in several LBM variables and appMMI (all P < 0.035). Serum hsIL-6, hsCRP and hsTNF-α changes did not reach significance. Baseline hsIL-6 and hsCRP levels were negatively correlated with several LBM variables but solely baseline hsIL-6 was associated with changes in appLBM. No correlations were found between changes in measured variables.
Discussion
Attenuation of eHSP72 following resistance training in parallel with increase in LBM variables showed a concordance between the evolution of this biomarker and a clinical outcome relevant to sarcopenia.
Conclusion
Nevertheless, the low bloodstream detection rate of eHSP72 in a sarcopenic otherwise healthy population might limit its use in clinical settings for now.

Similar content being viewed by others
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127(5 Suppl):990S–991S
Visser M (2009) Towards a definition of sarcopenia—results from epidemiologic studies. J Nutr Health Aging 13:713–716
Janssen I, Baumgartner RN, Ross R et al (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Hunter GR, McCarthy JP, Bamman MM (2004) Effects of resistance training on older adults. Sports Med 34:329–348
Fujita S, Volpi E (2006) Amino acids and muscle loss with aging. J Nutr 136(1 Suppl):277S–280S
Koopman R (2011) Dietary protein and exercise training in ageing. Proc Nutr Soc 70:104–113
American College of Sports Medicine, Thompson WR, Gordon NF et al (2010) ACSM’s guidelines for exercise testing and prescription. In: Chapter 4: Health-related physical fitness testing and interpretation, p 90, 8th edn. Wolters Kluwer, Philadelphia, pp xxi, p 380
Fry CS, Rasmussen BB (2011) Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci 4(3):260–268
Wieland HA, Michaelis M, Kirschbaum BJ et al (2005) Osteoarthritis—an untreatable disease? Nat Rev Drug Discovery 4:331–344. doi:10.1038/nrd1693
Nedergaard A, Karsdal MA, Sun S et al (2013) Serological muscle loss biomarkers: an overview of current concepts and future possibilities. J Cachexia Sarcopenia Muscle 4:1–17
Ogawa K, Kim HK, Shimizu T et al (2012) Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 17:349–359
Beyer I, Njemini R, Bautmans I et al (2012) Inflammation-related muscle weakness and fatigue in geriatric patients. Exp Gerontol 47:52–59
Macario AJ, Conway de Macario E (2005) Sick chaperones, cellular stress, and disease. New Engl J Med 353:1489–1501
Pockley AG (2002) Heat shock proteins, inflammation, and cardiovascular disease. Circulation 105:1012–1017
Terry DF, Wyszynski DF, Nolan VG et al (2006) Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mech Ageing Dev 127:862–868
Ogawa K, Sanada K, Machida S et al (2010) Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm 2010:171023. doi:10.1155/2010/171023
Aleman H, Esparza J, Ramirez FA et al (2011) Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing 40:469–475
Schaap LA, Pluijm SM, Deeg DJ et al (2006) Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119:526.e9–526.e17
Washburn RA, Smith KW, Jette AM et al (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Luhrmann PM, Herbert BM, Gaster C et al (1999) Validation of a self-administered 3-day estimated dietary record for use in the elderly. Eur J Nutr 38:235–240
Beyer I, Bautmans I, Njemini R et al (2011) Effects on muscle performance of NSAID treatment with piroxicam versus placebo in geriatric patients with acute infection-induced inflammation. A double blind randomized controlled trial. BMC Musculoskel Disord 12:292
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci 56:M146–M156
Chang SS, Weiss CO, Xue QL et al (2012) Association between inflammatory-related disease burden and frailty: results from the Women’s Health and Aging Studies (WHAS) I and II. Arch Gerontol Geriatr 54:9–15
Leng SX, Xue QL, Tian J et al (2007) Inflammation and frailty in older women. J Am Geriatr Soc 55(6):864–871
Walston J, McBurnie MA, Newman A et al (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162:2333–2341
Welc SS, Judge AR, Clanton TL (2013) Skeletal muscle interleukin-6 regulation in hyperthermia. Am J Physiol Cell Physiol 305(4):C406–C413
Yamada P, Amorim F, Moseley P et al (2008) Heat shock protein 72 response to exercise in humans. Sports Med 38:715–733
Fleshner M, Johnson JD (2005) Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 21:457–471
Whitham M, Fortes MB (2008) Heat shock protein 72: release and biological significance during exercise. Front Biosci J Virtual Libr 13:1328–1339
Morton JP, Kayani AC, McArdle A et al (2009) The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39:643–662
Acknowledgments
The authors would like to thank Martine Fisch, Christine Rioux-Perreault, Johann Lebon, Daniel Mireault, Joanie Lévesque and all kinesiologists supervising the exercise training protocol for their professional assistance. We are also grateful to all men who participated in this study. The Dairy Farmers of Canada supported this study financially. Karine Perreault detains a master degree scholarship from the Centre de recherche sur le vieillissement du CSSS-IUGS. Isabelle J. Dionne holds a Canada Research Chair in Exercise recommendations in healthy aging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Human and Animal Rights
All procedures performed in this study were in accordance with the ethical standards of the Research Ethics Committee of the CSSS-IUGS and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Informed consent
All participants signed a written informed consent during the first visit to the Research Centre on Aging (Geriatric Institute of the University of Sherbrooke, CSSS-IUGS, Sherbrooke, QC, Canada).
Rights and permissions
About this article
Cite this article
Perreault, K., Courchesne-Loyer, A., Fortier, M. et al. Sixteen weeks of resistance training decrease plasma heat shock protein 72 (eHSP72) and increase muscle mass without affecting high sensitivity inflammatory markers’ levels in sarcopenic men. Aging Clin Exp Res 28, 207–214 (2016). https://doi.org/10.1007/s40520-015-0411-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0411-7