Abstract
Adverse reactions to dental materials occur and public interest in this topic has increased during recent decades. Thus, improving the biocompatibility of dental materials is necessary and must be based on several strategies. First, a strategy for improving the administrative and technical conditions for material certification processes should be included, such as the development of in vitro tests with enhanced predictability of the generated data for use in the clinic. Second, research on material/tissue interactions must be enhanced and include mechanistic approaches, as this strategy leads to the development of new and more biocompatible materials. Research into patients and their individual exposure situation is a strategy directed at better defining risk groups. Finally, improvement of education will also lead to improved biocompatibility of dental materials.
Similar content being viewed by others
Introduction
Adverse reactions to dental materials occur in patients and dental personnel [1••]. Local reactions take place at the exposure site, e.g., dental pulp and pulp inflammation after pulp capping with adhesives and resin-based composites has been described [2–5], which was related to substances released from the applied materials. Also, inhibition of biomineralization after pulp capping with adhesives was observed [6] and can also be related to eluted substances from dental materials such as acrylic monomers [7, 8••].
In other cases, pulp inflammation has been related to bacteria under restorative materials such as resin-based composites [9, 10], and bacterial growth was found to be promoted by such materials [11]. Recurrent caries, a bacteria-mediated process, has been reported to be one of the most important reasons for restoration failures, especially with resin-based composites [12]. In these cases, bacteria-mediated adverse effects can be considered to be indirectly caused by the dental material.
Systemic reactions occur in tissues and organs distant from the exposure site and have mainly been discussed for amalgam [1••] in the past, but nowadays are also discussed regarding resin-based composites in respect to the content of bisphenol-A and its possible health effects [13]. Recently, the biologic effect of materials on the environment, e.g., mercury or bisphenol-A, have been under discussion [13–15]. Allergic reactions to resin-based composites and metals, such as nickel, palladium, cobalt, or gold, have been observed in patients and in dental personnel [1••, 16, 17••].
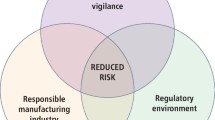
Clinically observed adverse reactions cover a broad spectrum of different clinical entities and are based on different mechanisms. Furthermore, these reactions are observed with a large number of different dental materials. This complex situation makes it plausible that (1) strategies to improve biocompatibility of dental materials are necessary; and (2) not one but several strategies for biocompatibility improvement of dental materials need to be pursued in parallel.
Definitions and Aims
A strategy can be defined as a human attempt to achieve desirable ends or aims with the available means [18]. In this context, the aim is to have biocompatible materials and devices available. What does this mean?
In 1987, biocompatibility was defined as the ability of a material to perform with an appropriate host response when applied as intended [19]. Later, this definition was considered to be too general. In 2008, Williams proposed that biocompatibility can be regarded as the ability of a biomaterial to perform its desired function with respect to a medical therapy without eliciting any clinically significant adverse effects in the recipient of that therapy, generating the most appropriate beneficial cellular or tissue response to that specific situation, and optimizing the clinically relevant performance of the therapy [19].
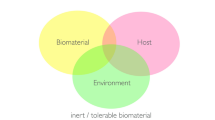
This definition covers different concepts of material/tissue interaction. The biotolerant/inert material concept is aimed at materials that do not harm tissues (see below) [19]. Further to this, the above definition covers bioactive materials, which are not meant to substantially degrade but are designed to stimulate biological effects, such as dentin bridge formation after pulp capping with MTA (mineral trioxide aggregate) or related materials [20, 21•]. Finally, the above definition includes materials used as scaffolds in tissue engineering for assisting in tissue regeneration. These materials must degrade, which is a further challenge in respect to the release of toxic moieties or of particles [19].
The term biocompatibility is closely related to the term safety; in this context, safety is defined as freedom from unacceptable risks [22]. Both of the terms biocompatibility and safety refer to the risk concept. In daily life, the term risk is well-known, but it is used mainly intuitively and is not related to the actual risk [23]. In science and within certification processes (see below), risk is defined as the combination of the severity of an adverse effect and the frequency of it occurring [22]. This realistically means that the strategic aim can only be to minimize adverse effects under the given clinical therapeutic situation, because freedom from any adverse effect occurring is virtually impossible.
Improvement of Biocompatibility by Consumer Protection
The aim of this strategy is to prevent damage for the patient, dental personnel, and environment that may potentially be derived from new materials or to test for new risks for known materials (for instance, bisphenol-A in resin-based composites). Thus, biocompatibility of dental materials on the market is improved through a preclinical certification process by eliminating the entrance of problematic materials into the market place.
Such certification processes are legally regulated worldwide and dental materials are classified as Medical Devices (used in dentistry). New materials must pass this certification process before they are allowed to be marketed [1••]. Here, an important aspect is biocompatibility.
The basis for biocompatibility evaluation is a Clinical Risk Assessment, which evaluates potential risks related to the material properties, due to the exposed tissue and the duration of exposure, mainly according to internationally recognized ISO (International Organization for Standardization) standards, such as ISO 14971 [22]. If necessary information is lacking, additional tests need to be performed. Again, ISO standards are applicable such as the ISO 10993 series for medical devices in general [1••] and ISO 7405, which is especially for medical devices used in dentistry [24]. The strategy for test selection after the Clinical Risk Assessment is based on the rule that in vitro tests are performed first, e.g., elution tests with a chemical analysis of eluted substances, followed by cytotoxicity and genotoxicity/mutagenicity tests. Then, if deemed necessary, animal experiments, e.g., for subchronic/chronic toxicity or for carcinogenicity, should be performed. For preclinical testing of a sensitizing potential, the maximization test on guinea pigs is mainly performed [25], although recently the LLNA (local lymph node assay) has become more and more popular [26].
After market approval, a post-market surveillance system, in which the user (e.g., the dentist) is obliged to report on adverse effects in his/her patients to the respective competent authorities, will further protect the consumer. In summary, in a series of different standardized tests certain phenomena/outcomes are measured and the data are assessed for predicting the clinical behavior of the material. Furthermore, clinically observed adverse effects (phenomena) are to be reported.
Has this strategy, which has been introduced worldwide in the past two decades, led to more biocompatible materials on the market? This question is difficult to answer. In any case, the awareness of producers (and the public) has increased. The success of this strategy depends on (1) the predictability of the preclinical data for the clinical situation; (2) the evaluation process and proper control by the involved third parties, such as the FDA in the US or the Notified Bodies in the EU; and (3) the appropriate use of the materials.
The recent discussion on failed silicone breast implants, which are subject to the same methods of certification as dental materials, raised doubts as to whether such certification programs are effective [27]. Therefore, both scientific and administrative/political processes are to be reconsidered. Presently, the medical device legislation is being revised within the EU administration, e.g., with the aim of more stringent control of the Notified Bodies or the introduction a unique device identifier (UDI) for all medical devices [27]. A further problem is the confidentiality of data submitted by the manufacturer, which does not allow for scientific control of the process.
Finally, test methods with better predictability must be developed and used in the certification process. As animal experiments are expensive, time consuming, and under public discussion, in vitro approaches have to be elaborated further by better adjusting the test conditions to the clinical situation. One example is the development of the dentin barrier test: here, a dentin slice of a defined thickness was incorporated into a cell culture-based test system because it is known that dentin modifies the pulp reaction to dental materials [28, 29]. Furthermore, three-dimensional cell cultures, which better simulate the in vivo conditions than do monolayer cell cultures, should be developed further and used. Methods are available elsewhere [30–32]. The Dentin-Barrier-Test has been incorporated into the ISO 7405 standard [24].
Improvement of Biocompatibility by a Mechanistic Analysis of Material Toxicity
The aim of this strategy is to elucidate the mechanisms behind the observed or tested adverse reactions (phenomena) elicited by dental materials, with the goal of improving the biocompatibility of materials [33]. For this strategy, standardized methods, such as those mentioned above, are not suitable, but individual scientific approaches are needed. Here, in vitro methods, which can specifically be designed to test special hypotheses, are often used and helpful. Biocompatibility of dental materials is mainly related on their surface characteristics and to substances eluted from the material into the tissue environment.
Biocompatibility Related to Material Surface Characteristics
Examples of surface characteristics are surface chemistry, surface topography (e.g., roughness, texture), wettability (contact angle) [34], surface charge [35••], or surface free energy [36]. The surface chemistry of a material per se influences, for example, the adhesion of cells and of bacteria or cell proliferation [37, 38]. The same was shown for surface topography (roughness, texture) [39], although the situation is rather complex and depends on the amount of roughness and the specific structures [37]. Furthermore, and most importantly, as soon as a biomaterial (including dental materials) comes into contact with body tissues, proteins from tissue fluids will immediately adsorb on the material surface. This process is determined by the composition of the tissue fluid and the above-mentioned material surface characteristics [34, 40••].
Protein adsorption itself changes the surface characteristics of the material, e.g., in one study the surface angle of hydrophilic surfaces was increased and those of hydrophobic surfaces decreased after material surface exposure to serum [38]. Also, material surface exposure to saliva before testing changed the contact angle in comparison with the “naked” surface [40••]. This protein layer may influence cell and bacterial adhesion. Compared to the untreated surfaces of hydrophilic (low adhesion) and hydrophobic surfaces (high adhesion), serum coating leveled off these differences for Staphylococcus aureus [38]. For Streptococcus gordonii, a negative correlation between the hydrophobicity of pure surfaces and the number of attached microorganisms was not any more observed after surfaces were coated with salivary proteins [40••]. This protein layer also influences the surface chemistry: in one study, the original bactericidal activity of pyridinium-coated cationic surfaces against S. gordonii and Streptococcus mutans was found to be greatly reduced upon adsorption of a protein film [35••]. Furthermore, there is evidence that bacterial adhesion to these surfaces is ligand mediated [41] and, again, the protein on the material will interact.
This all leads to the conclusion that protein–material interaction needs to be addressed; for example, the use of dendrimeric molecules with antibacterial groups, which are active despite the presence of a protein layer, was proposed recently [42]. Consequences for the development of new materials are that mechanistic approaches are needed to address surface effects on biocompatibility and their modification through protein adsorption, e.g., after contact with saliva.
Biocompatibility Related to Eluted Moieties
While cell death was formerly a commonly accepted endpoint for cytotoxicity tests [1••], new approaches were recently followed that studied in more detail the cytotoxicity and changes in the cell metabolism after exposure to low and non-lethal concentrations of substances from dental materials and differentiating these into necrosis or apoptosis [33]. This apparently better reflects the clinical exposure situation. In this context, cytotoxicity can be the result of (1) a direct interaction of the eluted substance with relevant cellular macromolecules; or (2) the formation of intracellular metabolic products, which then cause cellular reactions such as apoptosis (indirect interaction of substances with cells).
Direct interaction: examples are the carbonyl moieties of methacrylates adjacent to the carbon–carbon double-bond function, which may act as electron-withdrawing groups [43••, 44]. It was hypothesized that this positive charge of the beta carbon of the double bond can react with nucleophilic centers; for instance, amino or thiol groups in macromolecules (DNA or proteins), or thiol groups (-SH) in small cellular molecules such as glutathione (GSH) via Michael addition [43••, 44].
Indirect interactions [here through a reactive oxygen species (ROS) imbalance]: these have been investigated in more detail recently with different acrylic compounds. Apoptosis is commonly observed in cells after exposure to low concentrations of acrylic monomers and eluates from resin-based materials [33, 43••, 45, 46]. It has been shown that cell exposure to acrylic monomers such as HEMA (hydroxyethylmethacrylate) or TEGDMA (triethylenglycoledimethacrylate) leads to the intracellular depletion of GSH, a tripeptide synthesized from glycine, cysteine, and glutamate [47–49]. Interestingly, a simultaneous increase of GSSG—the oxidized form of GSH—was not detected [47, 50•]. Apparently, GSH detoxifies these monomers by conjugating their α,β-unsaturated carbon–carbon moiety to the thiol group, with the catalysis of GSH S-transferases (GST). This reaction determines a GSH cellular depletion and belongs to the metabolism of α,β-unsaturated esters, protecting the body against the toxic effects of electrophiles [51].
As GSH is also the major non-enzymatic antioxidant, concentration of ROS is increased (ROS imbalance), which has been shown for multiple target cells, several acrylic monomers, and resin-based material eluates [46, 47, 49, 52–54]. The amount of ROS exceeds the capacities of intracellular non-enzymatic and enzymatic antioxidant systems [43••]. This ROS imbalance leads to effects such as the following:
-
DNA oxidation with single- and double-strand breaks [55];
-
Eventually apoptosis, if the DNA damage is not repaired (see above);
-
Furthermore, MAP (mitogen-activated protein) kinases (stress kinases) are upregulated, although their role in this context is unclear [57–59];
-
Enzymatic systems such as GSH transferase, superoxide dismutase, or catalase are influenced. By upregulation of the catalase, the ROS imbalance (here, especially the formation of hydrogen peroxide) will be counteracted [60•];
-
Furthermore, low monomer concentrations and/or related substances inhibited odontoblast cell differentiation [61] and specific odontoblast functions including alkaline phosphatase activity, the matrix mineralizing capability, calcium deposition, and gene expression such as dentin sialoprotein [7, 8••, 62••].
This shows that acrylic monomers are environmental stressors [43••]. ROS imbalance has been found to be related to the toxicity of various other compounds and of UV radiation [33]. Thus, ROS imbalance can be regarded as a rather unspecific cell response to a variety of electrophilic stressors. Beside ROS, other cellular reactive species such as reactive nitrogen species (RNS) may also influence cellular reactions in this context. However, to date, information for dental materials is scarce [43••].
Biocompatibility Mediated Through Bacteria
A more indirect mechanism of adverse reactions elicited by dental materials is related to bacteria accumulations, as shown above. Further to this, it was shown that acrylic monomers such as TEGDMA reduce the cellular immune system directed against bacteria. In macrophages, cell-surface relevant antigens and proinflammatory cytokines were upregulated after exposure to lipopolysaccharide, but TEGDMA caused a significant downregulation [63]. This shows that the acrylic monomer may decrease bacterial clearance because it blocks the inflammation process [64].
Improvement of Biocompatibility Through New Materials
Based on information derived from mechanistic studies (see above), new materials can be developed. Generally, it should be aimed at reducing (1) the amount of eluted substances, e.g., by better polymerization (higher degree of conversion); and (2) the inherent toxicity of material ingredients. For instance, oxiranes and siloranes were available for low-shrinking ring-opening resin molecules: the mutagenic effect of oxiranes in contrast to siloranes led to the further development of the siloranes [65, 66]. This also means that biocompatibility tests should be done at an early stage of new material development, preferably on the ingredients.
Use of Antioxidants
As previously mentioned, ROS imbalance can be regarded as a rather unspecific cell response to exposure from different materials and it is responsible for different cellular reactions such as apoptosis or blocking biomineralization. Thus, whether antioxidants could prevent such reactions has been frequently tested. A powerful substance in this context was N-acetyl-cysteine (NAC) [8••, 43••, 46, 57, 67]. NAC may act through different mechanisms:
-
It may form intracellular adducts with the test compound, thus reducing its effective intracellular concentration, as was shown for HEMA [68];
-
NAC itself is a scavenger of free radicals and thus may reduce the ROS concentration [8••];
-
NAC is also a precursor of GSH, and thus the GSH concentration is increased and the antioxidant capacity of cells enhanced [8••, 43••].
NAC could possibly be applied in two ways: (1) as a separate agent before the application of the dental material; or (2) as an addition to the dental material, thus being applied together with it.
NAC application before cell exposure to acrylic substances has been performed in cell culture experiments and it was consistently shown that the ROS concentration and apoptosis rate declined and biomineralization improved [43••, 60•]. However, a clinical protocol has not yet been developed, nor is there information available on the applicability of such a procedure.
Regarding the second possible application, the inclusion of NAC into the dental material, the addition of NAC into poly(methyl)methacrylate materials was tested by Kojima et al. [69] in odontoblast-like dental pulp cells and by Tsukimura et al. [70] in osteoblasts. The protective effect of NAC was clearly demonstrated. Similar results were shown by the same group after the addition of NAC to a Resin Modified Glass Ionomer Cement (RMGIC) [8••]. In contrast, the addition of substances to existing formulations may influence their physical and mechanical properties. In particular, some radical-based polymerization systems may be influenced by radical scavengers such as NAC. Information on this aspect is scarce so far.
Other antioxidants such as vitamins C, E, and A were tested; vitamins C and E were dose-dependently protective against HEMA and TEGDMA toxicity in two cell lines, vitamin A only in one cell line, and the hydrophilic vitamin C was the most effective [71]. Trolox (a water-soluble analog of vitamin E) and ascorbate (a reduced form of vitamin C) greatly inhibited TEGDMA-induced cytotoxicity and GSH depletion. Trolox acts as a chain-breaking antioxidant by quenching free radicals and interrupting lipid peroxidation [47]. As early as 1975, Antonucci et al. [72] reported on new types of initiator systems in cold-curing methacrylates by employing, for example, ascorbic acid as the accelerator; these types of systems should offer promise for improving storage, color stabilities, and biocompatibility. On the other side, combinations of ascorbic acid (vitamin C) and vitamin E (α-tocopherol) did not prevent DNA damage induced by camphorquinone and dimethyl-p-toluidine [73]. In summary, there are some indications for the use of other antioxidants in dental materials, but the data are less clear than for NAC.
Antibacterial Substances in Dental Materials
The incorporation of antibacterial substances into dental materials has been practiced for many decades. Classically, substances such as metals (copper, zinc, or silver), fluorides, cetylpyridiniumchlorid, different chlorhexidine compounds, glutaraldehyde, triclosan, zinc oxide, and eugenol, and even antibiotics, haven been included or tested for antibacterial activity. Their antibacterial effect mainly depends upon the continued release of the antibacterial substance from the material or upon a “recharging” and then a release, as was proposed for the fluorides in glass ionomer cements [74].
A different approach was chosen by Imazato and co-workers [75, 76••], who added an antibacterial monomer [MDPB (12-methacryloyloxydodecylpyridinium bromide)] to the base monomers. After setting, the antibacterial monomer was tightly bound to the polymer network. New approaches are the use of dendrimers for creating bacteria-repellant surfaces. Laboratory data are promising [42] but patient data are missing. Another comparatively new approach is the incorporation of nanoparticles/microparticles, e.g., from silver [77–80] or zinc [78], into dental materials. In laboratory testing, antibacterial distant and repellent (surface) effects have been found [77–80].
However, all these approaches are somewhat problematic, as discussed below.
-
First of all, the physical/mechanical properties of materials may be negatively influenced by the incorporation and subsequent elution of antibacterial substances such as silver [81••]; although, apparently, formulations are available without impairment of physical and mechanical properties [82].
-
Furthermore, it is questionable whether the amount of substances released—which normally decreases with time—is sufficient to elicit a clinically relevant effect, as, for example, was discussed for the fluoride released from glass ionomer cements [83, 84].
-
Addition of antibacterial substances may also increase the release of other substances from the materials, as was shown for the incorporation nanosilver, which led to an increased monomer release [85].
-
The possible inactivation of antibacterial surface properties through proteins from saliva has already been discussed above [35••]. Another interaction of the antibacterial substances such as acidic (and therefore antibacterial) adhesives with dentin has to be considered: the neutralization of the acid after contact with dentin and its subsequent biological inactivation (unpublished data).
-
Finally, it should be kept in mind that most antibacterial substances used in dental materials act through rather unspecific mechanisms (with the exception of antibiotics) and, therefore, the action against bacteria implies a similar reaction against cells (i.e., cytotoxicity).
A possible solution could be a time limitation of the antibacterial action, e.g., through the use of antibacterial substances that can be regulated by light [86, 87•, 88]. This would also take care of other concerns that refer to the possibility of changing the oral ecoystem by a constant release of antibacterial substances with unclear consequences.
Finally, despite a large number of laboratory studies showing antibacterial properties, the effect in the clinic remains to be determined. For instance, a Cochrane database report was unable to identify any randomized controlled trials on the effects of antibacterial agents incorporated into resin composite restorations for the prevention of dental caries [89].
Summarizing the available data it becomes clear that the inclusion of antibacterial substances seems to be a promising strategy for improving the biocompatibility of dental materials. However, despite many approaches over the years, so far no real breakthrough has been observed. A new approach could indeed be the use of light-regulated antibacterial substances.
Improvement of Biocompatibility by Risk Group Identification
The aim of the risk group identification strategy is to define specific parts of the general population in which, because of special predispositions, higher exposure or other circumstances have or may have a higher risk for the development of adverse effects against specific materials. Avoidance of exposure to this specific material for this community may result in avoidance of clinical adverse reactions, and thus the overall biocompatibility of special dental materials is improved.
This is a well-known strategy and has been successfully applied for many years for patients with allergies [1••]. Pregnant and lactating females have been advocated to be a special risk group (including the fetus) for amalgam (see Schmalz and Arenholt-Bindslev [1••]); however, evaluations of the current evidence by national and international government commissions have not resulted in specific restrictions [1••]. Nevertheless, as extensive dental treatment in pregnant women may generally create a stressful situation, the German national administration has, together with scientific and professional dental associations, recommended that extensive dental treatment (i.e., except for emergency treatment) during pregnancy should, in general, be avoided [90]. No such risks are described for lactating women. Children were also a candidate population for an increased risk for adverse effects related to mercury from amalgam, e.g., due to the developing brain [91, 92]. However, in two independent clinical studies no such effects could be shown [91, 92].
Dental personnel are a risk group due to the high exposure to dental materials, especially in an uncured state. A no-touch technique for dental materials is recommended and regulations for safety at the working environment need to be followed [1••].
New strategies for defining risk groups have been proposed to be related to the genetic makeup of special patient/population groups. It has been suggested that polymorphisms in selenoproteins and GSH-related genes may influence elimination of mercury [93], and data from an Amazon population indicate that some GSH-related polymorphisms, such as GSTM1 and GCLM, may modify methyl mercury metabolism and mercury-related antioxidant effects [94]. Enzymatic (e.g., catalase) and non-enzymatic (GSH) cellular antioxidant systems have been shown to also be necessary for compensating the increase of cellular ROS after dental methacrylate monomer exposure [43••, 60•]. Furthermore, the influence of different variants of GSH transferase on the cellular reactions towards resin monomers was reported [95]. Altogether, scientific evidence for the impact of the genetic makeup on the exposure to substances from materials is still very weak. Furthermore, no tests are available for identification of such risk groups. However, this is apparently a very attractive field for further research and is a potential new strategy for improving dental material biocompatibility in the population.
Improvement of Biocompatibility by Training Dental Personnel
As is known from dental material science, the properties of the materials also depend on the correct handling by the dental technician or dentist. An example of incorrect handling by the dental technician is the insufficient removal of the metal oxide layer that develops on the metal surface after firing of a metal ceramic crown from all parts of the metal that are not covered by ceramic; the consequence is a gingivitis at the crown margin [1••, 16]. An example of incorrect handling by the dentist is the incorrect positioning of the light-curing lamp for polymerizing resin-based composites. This may lead to lip burns [96] or to insufficiently cured resin [97••]; insufficient cure leads to increased elution [98] and increased cytotoxicity [99]. Thus, improvement of dental education and the development of suitable tools [97••] can also be regarded as a strategy for improvement of biocompatibility of dental materials.
Conclusions
Several strategies have to be followed to improve the biocompatibility of dental materials (see Table 1) and they must be directed at different addressees: administrators, university- and industry-based researchers, and university teachers. As bioactive materials and scaffold materials for tissue engineering will increasingly become the center of interest for material development in the future, the importance of biocompatibility aspects in material assessment will also increase.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schmalz G, Arenholt-Bindslev D. Biocompatibility of dental materials. Heidelberg: Springer; 2009. Provides an overview on the whole field.
Dammaschke T, Stratmann U, Fischer RJ, Sagheri D, Schafer E. A histologic investigation of direct pulp capping in rodents with dentin adhesives and calcium hydroxide. Quintessence Int. 2010;41:e62–71.
Accorinte ML, Loguercio AD, Reis A, Costa CA. Response of human pulps capped with different self-etch adhesive systems. Clin Oral Investig. 2008;12:119–27.
Nowicka A, Parafiniuk M, Lipski M, Lichota D, Buczkowska-Radlinska J. Pulpo-dentin complex response after direct capping with self-etch adhesive systems. Folia Histochem Cytobiol. 2012;50:565–73.
Pameijer CH, Stanley HR. The disastrous effects of the “total etch” technique in vital pulp capping in primates. Am J Dent 1998;11 Spec No:S45–S54.
Tziafas D, Koliniotou-Koumpia E, Tziafa C, Papadimitriou S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. Int Endod J. 2007;40:58–66.
Galler KM, Schweikl H, Hiller KA, Cavender AC, Bolay C, D'Souza RN, et al. TEGDMA reduces mineralization in dental pulp cells. J Dent Res. 2011;90:257–62.
Minamikawa H, Yamada M, Iwasa F, Ueno T, Deyama Y, Suzuki K, et al. Amino acid derivative-mediated detoxification and functionalization of dual cure dental restorative material for dental pulp cell mineralization. Biomaterials. 2010;31:7213–25. One of the first approaches to include NAC in a filling material.
Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467–80.
Splieth C, Bernhardt O, Heinrich A, Bernhardt H, Meyer G. Anaerobic microflora under Class I and Class II composite and amalgam restorations. Quintessence Int. 2003;34:497–503.
Hansel C, Leyhausen G, Mai UE, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60–7.
Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children's Amalgam Trial. J Am Dent Assoc. 2007;138:763–72.
Goldberg M, Dimitrova-Nakov S, Schmalz G. BPA from dental resin material: where are we going with restorative and preventive dental biomaterials? Clin Oral Investig. 2014;18:347–9.
Arenholt-Bindslev D. Environmental aspects of dental filling materials. Eur J Oral Sci. 1998;106:713–20.
Fan PL, Arenholt-Bindslev D, Schmalz G, Halbach S, Berendsen H. Environmental issues in dentistry--mercury. FDI Commission. Int Dent J. 1997;47:105–9.
Garhammer P, Schmalz G, Hiller KA, Reitinger T, Stolz W. Patients with local adverse effects from dental alloys: frequency, complaints, symptoms, allergy. Clin Oral Investig. 2001;5:240–9.
Schmalz G, Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002;18:396–406. This paper provides an overview on metal toxicity for dental alloys.
Wikipedia. Strategy. http://en.wikipedia.org/wiki/Strategy. Accessed 6 Jul 2014.
Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–53.
Dammaschke T, Wolff P, Sagheri D, Stratmann U, Schafer E. Mineral trioxide aggregate for direct pulp capping: a histologic comparison with calcium hydroxide in rat molars. Quintessence Int. 2010;41:e20–30.
Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. 2012;91:1166–71. This study shows the biological interaction of new bioactive materials with the tissue.
International Organization for Standardization. ISO 14971:2007 medical devices - application of risk management to medical devices. Geneva: International Organization for Standardization; 2007.
Fisher E, Achilles S, Tonnies H. Predictive genetic testing, risk communication, and risk perception: an international expert meeting in Berlin, Germany. J Community Genet. 2014;5:1–5.
International Organization for Standardization. ISO 7405: Dentistry. Preclinical evaluation of the biocompatibility of medical devices used in dentistry: test methods for dental materials. Geneva: International Organization for Standardization; 2012 2013.
International Organization for Standardization. ISO 10993-10:2010 biological evaluation of medical devices Part 10: tests for sensitization and irritation. Geneva: International Organization for Standardization; 2010.
Gerberick GF, Ryan CA, Dearman RJ, Kimber I. Local lymph node assay (LLNA) for detection of sensitization capacity of chemicals. Methods. 2007;41:54–60.
Parvizi N, Woods K. Regulation of medicines and medical devices: contrasts and similarities. Clin Med. 2014;14:6–12.
Galler K, Hiller KA, Ettl T, Schmalz G. Selective influence of dentin thickness upon cytotoxicity of dentin contacting materials. J Endod. 2005;31:396–9.
Schmalz G, Schuster U, Koch A, Schweikl H. Cytotoxicity of low pH dentin-bonding agents in a dentin barrier test in vitro. J Endod. 2002;28:188–92.
Schmalz G, Schuster U, Nuetzel K, Schweikl H. An in vitro pulp chamber with three-dimensional cell cultures. J Endod. 1999;25:24–9.
Schuster U, Schmalz G, Thonemann B, Mendel N, Metzl C. Cytotoxicity testing with three-dimensional cultures of transfected pulp-derived cells. J Endod. 2001;27:259–65.
Schmalz G, Gröppel F, Hiller K-A, Galler KM. Three dimensional human cell cultures for cytotoxicity testing of dental filling materials. Acta Stomatol Croat. 2014;48:99–108.
Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res. 2006;85:870–7.
Muller R, Hiller KA, Schmalz G, Ruhl S. Chemiluminescence-based detection and comparison of protein amounts adsorbed on differently modified silica surfaces. Anal Biochem. 2006;359:194–202.
Muller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, et al. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. A study showing the effect of protein surface adsorption on antibacterial properties.
Herbst D, Dullabh H, Sykes L, Vorster C. Evaluation of surface characteristics of titanium and cobalt chromium implant abutment materials. SADJ. 2013;68(350):352–6.
Schweikl H, Muller R, Englert C, Hiller KA, Kujat R, Nerlich M, et al. Proliferation of osteoblasts and fibroblasts on model surfaces of varying roughness and surface chemistry. J Mater Sci Mater Med. 2007;18:1895–905.
Muller R, Ruhl S, Hiller KA, Schmalz G, Schweikl H. Adhesion of eukaryotic cells and Staphylococcus aureus to silicon model surfaces. J Biomed Mater Res A. 2008;84:817–27.
Bigerelle M, Anselme K. Statistical correlation between cell adhesion and proliferation on biocompatible metallic materials. J Biomed Mater Res A. 2005;72:36–46.
Schweikl H, Hiller KA, Carl U, Schweiger R, Eidt A, Ruhl S, et al. Salivary protein adsorption and Streptococccus gordonii adhesion to dental material surfaces. Dent Mater. 2013;29:1080–9. A study showing the effect of protein surface adsorption on antibacterial properties.
Walz A, Stuhler K, Wattenberg A, Hawranke E, Meyer HE, Schmalz G, et al. Proteome analysis of glandular parotid and submandibular-sublingual saliva in comparison to whole human saliva by two-dimensional gel electrophoresis. Proteomics. 2006;6:1631–9.
Eichler M, Katzur V, Scheideler L, Haupt M, Geis-Gerstorfer J, Schmalz G, et al. The impact of dendrimer-grafted modifications to model silicon surfaces on protein adsorption and bacterial adhesion. Biomaterials. 2011;32:9168–79.
Krifka S, Spagnuolo G, Schmalz G, Schweikl H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials. 2013;34:4555–63. Overview of recent findings regarding the influence of methacrylates on cell metabolism.
Schweikl H, Schmalz G. Triethylene glycol dimethacrylate induces large deletions in the hprt gene of V79 cells. Mutat Res. 1999;438:71–8.
Spagnuolo G, Galler K, Schmalz G, Cosentino C, Rengo S, Schweikl H. Inhibition of phosphatidylinositol 3-kinase amplifies TEGDMA-induced apoptosis in primary human pulp cells. J Dent Res. 2004;83:703–7.
Krifka S, Seidenader C, Hiller KA, Schmalz G, Schweikl H. Oxidative stress and cytotoxicity generated by dental composites in human pulp cells. Clin Oral Investig. 2012;16:215–24.
Stanislawski L, Lefeuvre M, Bourd K, Soheili-Majd E, Goldberg M, Perianin A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res A. 2003;66:476–82.
Volk J, Engelmann J, Leyhausen G, Geurtsen W. Effects of three resin monomers on the cellular glutathione concentration of cultured human gingival fibroblasts. Dent Mater. 2006;22:499–505.
Chang HH, Guo MK, Kasten FH, Chang MC, Huang GF, Wang YL, et al. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials. 2005;26:745–53.
Samuelsen JT, Kopperud HM, Holme JA, Dragland IS, Christensen T, Dahl JE. Role of thiol-complex formation in 2-hydroxyethyl- methacrylate-induced toxicity in vitro. J Biomed Mater Res A. 2011;96:395–401. Interesting study on the influence of methacrylates on cell metabolism, especially on antioxidative systems.
Nocca G, Ragno R, Carbone V, Martorana GE, Rossetti DV, Gambarini G, et al. Identification of glutathione-methacrylates adducts in gingival fibroblasts and erythrocytes by HPLC-MS and capillary electrophoresis. Dent Mater. 2011;27:e87–98.
Spagnuolo G, Annunziata M, Rengo S. Cytotoxicity and oxidative stress caused by dental adhesive systems cured with halogen and LED lights. Clin Oral Investig. 2004;8:81–5.
Spagnuolo G, D'Anto V, Cosentino C, Schmalz G, Schweikl H, Rengo S. Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27:1803–9.
Demirci M, Hiller KA, Bosl C, Galler K, Schmalz G, Schweikl H. The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent Mater. 2008;24:362–71.
Eckhardt A, Gerstmayr N, Hiller KA, Bolay C, Waha C, Spagnuolo G, et al. TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials. 2009;30:2006–14.
Schweikl H, Hartmann A, Hiller KA, Spagnuolo G, Bolay C, Brockhoff G, et al. Inhibition of TEGDMA and HEMA-induced genotoxicity and cell cycle arrest by N-acetylcysteine. Dent Mater. 2007;23:688–95.
Krifka S, Petzel C, Hiller KA, Frank EM, Bosl C, Spagnuolo G, et al. Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials. 2010;31:2964–75.
Krifka S, Hiller KA, Bolay C, Petzel C, Spagnuolo G, Reichl FX, et al. Function of MAPK and downstream transcription factors in monomer-induced apoptosis. Biomaterials. 2012;33:740–50.
Samuelsen JT, Dahl JE, Karlsson S, Morisbak E, Becher R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater. 2007;23:34–9.
Krifka S, Hiller KA, Spagnuolo G, Jewett A, Schmalz G, Schweikl H. The influence of glutathione on redox regulation by antioxidant proteins and apoptosis in macrophages exposed to 2-hydroxyethyl methacrylate (HEMA). Biomaterials. 2012;33:5177–86. Study highlighting the role of GSH.
About I, Camps J, Mitsiadis TA, Bottero MJ, Butler W, Franquin JC. Influence of resinous monomers on the differentiation in vitro of human pulp cells into odontoblasts. J Biomed Mater Res. 2002;63:418–23.
Bakopoulou A, Leyhausen G, Volk J, Koidis P, Geurtsen W. Effects of resinous monomers on the odontogenic differentiation and mineralization potential of highly proliferative and clonogenic cultured apical papilla stem cells. Dent Mater. 2012;28:327–39. This study shows the influence of methacrylates on cell differentiation.
Eckhardt A, Harorli T, Limtanyakul J, Hiller KA, Bosl C, Bolay C, et al. Inhibition of cytokine and surface antigen expression in LPS-stimulated murine macrophages by triethylene glycol dimethacrylate. Biomaterials. 2009;30:1665–74.
Schmalz G, Krifka S, Schweikl H. Toll-like receptors, LPS, and dental monomers. Adv Dent Res. 2011;23:302–6.
Schweikl H, Schmalz G, Weinmann W. Mutagenic activity of structurally related oxiranes and siloranes in Salmonella typhimurium. Mutat Res. 2002;521:19–27.
Schweikl H, Schmalz G, Weinmann W. The induction of gene mutations and micronuclei by oxiranes and siloranes in mammalian cells in vitro. J Dent Res. 2004;83:17–21.
Paranjpe A, Cacalano NA, Hume WR, Jewett A. N-acetylcysteine protects dental pulp stromal cells from HEMA-induced apoptosis by inducing differentiation of the cells. Free Radic Biol Med. 2007;43:1394–408.
Nocca G, D'Anto V, Desiderio C, Rossetti DV, Valletta R, Baquala AM, et al. N-acetyl cysteine directed detoxification of 2-hydroxyethyl methacrylate by adduct formation. Biomaterials. 2010;31:2508–16.
Kojima N, Yamada M, Paranjpe A, Tsukimura N, Kubo K, Jewett A, et al. Restored viability and function of dental pulp cells on poly-methylmethacrylate (PMMA)-based dental resin supplemented with N-acetyl cysteine (NAC). Dent Mater. 2008;24:1686–93.
Tsukimura N, Yamada M, Aita H, Hori N, Yoshino F, Chang-Il LM, et al. N-acetyl cysteine (NAC)-mediated detoxification and functionalization of poly(methyl methacrylate) bone cement. Biomaterials. 2009;30:3378–89.
Walther UI, Siagian II, Walther SC, Reichl FX, Hickel R. Antioxidative vitamins decrease cytotoxicity of HEMA and TEGDMA in cultured cell lines. Arch Oral Biol. 2004;49:125–31.
Antonucci JM, Grams CL, Termini DJ. New initiator systems for dental resins based on ascorbic acid. J Dent Res. 1979;58:1887–99.
Lee S, Pagoria D, Raigrodski A, Geurtsen W. Effects of combinations of ROS scavengers on oxidative DNA damage caused by visible-light-activated camphorquinone/N, N-dimethyl-p-toluidine. J Biomed Mater Res B Appl Biomater. 2007;83:391–9.
Seppa L, Forss H, Ogaard B. The effect of fluoride application on fluoride release and the antibacterial action of glass ionomers. J Dent Res. 1993;72:1310–4.
Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–72.
Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–9. Interesting overview of antibacterial substances for dental materials.
Burgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, Handel G, et al. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol. 2009;54:595–601.
Kasraei S, Sami L, Hendi S, Alikhani MY, Rezaei-Soufi L, Khamverdi Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 2014;39:109–14.
Zhang K, Li F, Imazato S, Cheng L, Liu H, Arola DD, et al. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater. 2013;101:929–38.
Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013;29:450–61.
Sodagar A, Kassaee MZ, Akhavan A, Javadi N, Arab S, Kharazifard MJ. Effect of silver nano particles on flexural strength of acrylic resins. J Prosthodont Res. 2012;56:120–4. This study addresses the influence of silver nanoparticles on material properties.
Chen L, Shen H, Suh BI. Antibacterial dental restorative materials: a state-of-the-art review. Am J Dent. 2012;25:337–46.
Forss H, Nase L, Seppa L. Fluoride concentration, mutans streptococci and lactobacilli in plaque from old glass ionomer fillings. Caries Res. 1995;29:50–3.
van Dijken JW, Kalfas S, Litra V, Oliveby A. Fluoride and mutans streptococci levels in plaque on aged restorations of resin-modified glass ionomer cement, compomer and resin composite. Caries Res. 1997;31:379–83.
Durner J, Stojanovic M, Urcan E, Hickel R, Reichl FX. Influence of silver nano-particles on monomer elution from light-cured composites. Dent Mater. 2011;27:631–6.
Cieplik F, Spath A, Leibl C, Gollmer A, Regensburger J, Tabenski L, Hiller KA, Maisch T, Schmalz G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Investig 2013.
Cieplik F, Spath A, Regensburger J, Gollmer A, Tabenski L, Hiller KA, et al. Photodynamic biofilm inactivation by SAPYR–an exclusive singlet oxygen photosensitizer. Free Radic Biol Med. 2013;65:477–87. Describes new antibacterial substances with light activation.
Spath A, Leibl C, Cieplik F, Lehner K, Regensburger J, Hiller KA, et al. Improving photodynamic inactivation of bacteria in dentistry: highly effective and fast killing of oral key pathogens with novel tooth-colored type-II photosensitizers. J Med Chem. 2014;57:5157–68.
Pereira-Cenci T, Cenci MS, Fedorowicz Z, Azevedo M. Antibacterial agents in composite restorations for the prevention of dental caries. Cochrane Database Syst Rev. 2013;12, CD007819.
Harhammer R. Zur Risikobewertung des zahnärztlichen Füllungswerkstoffes Amalgam. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2001;44:149–54.
DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitão J, et al. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295(15):1784–92.
Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, et al. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295(15):1775–83.
Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals. Toxicol Appl Pharmacol. 2011;257:301–8.
Barcelos GR, Grotto D, de Marco KC, Valentini J, Lengert A, de Oliveira AA, et al. Polymorphisms in glutathione-related genes modify mercury concentrations and antioxidant status in subjects environmentally exposed to methylmercury. Sci Total Environ. 2013;463–464:319–25.
Lefeuvre M, Bourd K, Loriot MA, Goldberg M, Beaune P, Perianin A, et al. TEGDMA modulates glutathione transferase P1 activity in gingival fibroblasts. J Dent Res. 2004;83:914–9.
Spranley TJ, Winkler M, Dagate J, Oncale D, Strother E. Curing light burns. Gen Dent. 2012;60:e210–4.
Price RB, Strassler HE, Price HL, Seth S, Lee CJ. The effectiveness of using a patient simulator to teach light-curing skills. J Am Dent Assoc. 2014;145:32–43. This study demonstrates the influence of handling on material properties very well.
Pearson GJ, Longman CM. Water sorption and solubility of resin-based materials following inadequate polymerization by a visible-light curing system. J Oral Rehabil. 1989;16:57–61.
Sigusch BW, Pflaum T, Volpel A, Schinkel M, Jandt KD. The influence of various light curing units on the cytotoxicity of dental adhesives. Dent Mater. 2009;25:1446–52.
Compliance with Ethics Guidelines
Conflict of Interest
Gottfried Schmalz declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
ᅟ
No Human or Animal Studies Performed by the Authors
This article does not contain any studies with human or animal subjects performed by any of the authors.
Human Studies Done by Authors (But No Animal Studies)
This article does not contain any studies with animal subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Animal Studies Done by Authors (But No Human Studies)
This article does not contain any studies with human subjects performed by any of the authors.
With regard to the authors’ research cited in this paper, all institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmalz, G. Strategies to Improve Biocompatibility of Dental Materials. Curr Oral Health Rep 1, 222–231 (2014). https://doi.org/10.1007/s40496-014-0028-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40496-014-0028-5