Abstract
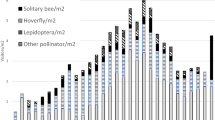
Fire is considered a major factor in the succession process in “Cerrado”, determining the dynamics and composition of these Neotropical savanna plant formations. However, when savannas are protected from fire, both fire-tolerant and fire-sensitive plant species increase in density and size, especially species of trees. Nevertheless, how this change in the vegetation structure relates to changes in the ecosystem function has been seldom evaluated. Pollination is a major ecosystem service, and here we aimed to determine possible changes in floristic composition, floral biology, and pollination systems in an area of open savanna protected against fire over a period of 20 years in Minas Gerais state, Central Brazil. The comparison over the two decades showed that ecological succession, in the absence of fire, increased the diversity and decreased the dominance of plant species. There was an increase in specie richness from 156 spp. in 1992 to 206 spp. in 2012, with appearance of trees and species with specialized pollination systems. Plants were predominantly pollinated by bees in both periods, but floral diversity and specialization seem to have increased after fire suppression, for instance, with the emergence of more species pollinated by moths. Not only generalist light-colored actinomorphic flowers, but also tubular or cup-like nectar flowers commonly associated with more specialized pollinators predominated in both periods. The dominance of these contrasting features actually increased after two decades, especially when we compare the number of individuals in each group. However, it seems that the increase in plant diversity and density of woody species did not lead to marked specialization of floral features and pollination system along the studied period.




Similar content being viewed by others
References
Albrecht M, Riesen M, Schmid B (2010) Plant–pollinator network assembly along the chronosequence of a glacier foreland. Oikos 119:1610–1624
Amorim FW, Wyatt GE, Sazima M (2014) Low abundance of long-tongued pollinators leads to pollen limitation in four specialized hawkmoth-pollinated plants in the Atlantic Rain forest, Brazil. Naturwissenschaften 101:893–905
Arantes CS, Vale VS, Oliveira AP, Junior JAP, Lopes SF, Schiavini I (2014) Forest species colonizing cerrado open areas: distance and area effects on the nucleation process. Braz J Bot 37:143–150
Backéus I (1992) Distribution and vegetation dynamics of humid savannas in Africa and Asia. J Veg Sci 3:45–356
Barbosa AAA (1996) Biologia reprodutiva de uma comunidade de campo sujo de cerrado, Uberlândia/MG. Doctoral Thesis, Universidade Estadual de Campinas, Campinas
Barbosa AAA, Sazima M (2008) Biologia reprodutiva de plantas herbáceo-arbustivas de uma área de campo sujo de cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Embrapa Cerrados, Planaltina, pp 293–318
Bawa KS (1990) Plant-pollinator interactions in a tropical rain forest. Ann Rev Ecol Syst 21:399–422
Bawa KS, Perry DR, Beach JH (1985) Reproductive biology of tropical lowland rain forest trees. I. Sexual systems and incompatibility mechanisms. Am J Bot 72:331–345
Begon M, Harper JL, Towsend CR (2009) Ecologia: de indivíduos a ecossistemas. Artmed, Porto Alegre
Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N (2007) Specialization, constraints, and conflicting interests in mutualistic networks. Curr Biol 17:41–346
Borges HBN (2000) Biologia reprodutiva e conservação do estrato lenhoso numa comunidade do Cerrado. Doctoral thesis, Universidade Estadual de Campinas, Campinas
Bremer B, Bremer K, Chase M, Fay M, Reveal J, Soltis D, Soltis P, Stevens P (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Brower JE, Zar JH, von Ende CN (1998) Field and laboratory methods for general ecology. WCB/McGraw-Hill, New York
Brown J, York A, Christie F, McCarthy M (2016) Effects of fire on pollinators and pollination. J Appl Ecol. doi:10.1111/1365-2664.12670
Cardoso E, Moreno MIC, Bruna EM, Vasconcelos HL (2009) Mudanças fitofisionômicas no Cerrado: 18 anos de sucessão ecológica na Estação Ecológica do Panga, Uberlândia-MG. Caminhos de Geografia (Uberlandia) 32:254–268
Coutinho LM (1977) Aspectos ecológicos do fogo no cerrado. II—as queimadas e a dispersão de sementes em algumas espécies anemocóricas do estrato herbáceo subarbustivo. Bol Bot USP 5:57–64
Coutinho LM (1990) Fire in the ecology of the Brazilian Cerrado. In: Goldhammer JG (ed) Fire in the tropical biota. Springer, Berlin, pp 82–105
Coutinho LM (2002) O bioma do cerrado. In: Klein AL (ed) Eugene warming e o cerrado brasileiro: um século depois. Editora da Unesp, São Paulo, pp 77–91
Deus FF, Vale VS, Schiavini I, Oliveira PE (2014) Diversity of reproductive ecological groups in semideciduous seasonal forests. Biosci J 30:1885–1902
Durigan G, Ratter JA (2006) Successional changes in cerradão and cerrado/forest ecotonal vegetation in western São Paulo State, Brazil, 1962–2000. Edinb J Bot 63:119–130
Durigan G, Siqueira MF, Franco GADC, Bridgewater S, Ratter JA (2003) The vegetation of priority areas for cerrado conservation in São Paulo State, Brazil. Edinb J Bot 60:217–241
Ebeling A, Klein AM, Schumacher J, Weisser WW, Tscharntke T (2008) How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 117:1808–1815
Felfili JM, Rezende AV, Silva Júnior MC, Silva MA (2000) Changes in floristic composition of cerrado sensu stricto in Brazil over a 9-year period. J Trop Ecol 16:576–590
Felfili JM, Silva Júnior MC, Sevilha AC, Fagg CW, Walter BMT, Nogueira PE, Rezende AV (2004) Diversity, floristic and structural patterns of cerrado vegetation in Central Brazil. Plant Ecol 175:37–46
Fensham RJ, Butler DW (2003) Spatial pattern of dry rainforest colonizing unburnt Eucalyptus savanna. Aust Ecol 28:121–128
Fensham RJ, Fairfax RJ, Butler DW, Bowman DMJS (2003) Effects of fire and drought in a tropical eucalypt savanna colonized by rain forest. J Biogeogr 30:1405–1414
Ferreira C, Maruyama PK, Oliveira PE (2016) Convergence beyond flower morphology? Reproductive biology of hummingbird-pollinated plants in the Brazilian Cerrado. Plant Biol 18:316–324
Fontaine C, Dajoz I, Meriguet J, Loreau M (2005) Functional diversity of plant-pollinator interaction webs enhances the persistence of plant communities. PLoS Biol 4:129–135
Franco A (2005) Biodiversidade de forma e função: implicações ecofisiológicas das estratégias de utilização de água e luz em plantas lenhosas do Cerrado. In: Scariot A, Souza Silva JC, Felfili JM (eds) Cerrado: ecologia, biodiversidade e conservação. Ministério do Meio Ambiente, Brasília, pp 179–196
Gathmann A, Greiler HJ, Tscharntke T (1994) Trap-nesting bees and wasps colonizing set-aside fields: sucession and body size, management by cutting and sowing. Oecologia 98:8–14
Geiger EL, Gotsch SG, Damasco G, Haridasan M, Franco AC, Hoffmann WA (2011) Distinct roles of savanna and forest tree species in regeneration under fire suppression in a Brazilian savanna. J Veg Sci 22:312–321
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Gignoux J, Lahoreau G, Julliard R, Barot S (2009) Establishment and early persistence of tree seedlings in an annually burned savanna. J Ecol 97:484–495
Gouveia GP, Felfili JM (1998) Fenologia de comunidades de cerrado e de mata de galeria no Brasil Central. Rev Arvore 22:443–450
Grundel R, Jean RP, Frohnapple KJ, Glowacki GA, Scott PE, Pavlovic NB (2010) Floral and nesting resources, habitat structure, and fire influence bee distribution across an open-forest gradient. Ecol Appl 20:1678–1692
Hegland SJ, Totland O (2005) Relationships between species’ floral traits and pollinator visitation in a temperate grassland. Oecologia 145:586–594
Henriques RPB (2005) Influência da história, solo e fogo na distribuição e dinâmica das fitofisionomias no bioma do Cerrado. In: Scariot A, Sousa Silva JC, Felfili JM (eds) Cerrado: ecologia, biodiversidade e conservação. Ministério do Meio Ambiente, Brasília pp, pp 75–92
Henriques RPB, Hay JD (2002) Patterns and dynamics of plant populations. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 140–158
Hoffmann WA, Adasme R, Haridasan M, Carvalho M, Geiger EL, Pereira MAB, Gotsch SG, Franco AC (2009) Tree topkill, not mortality, governs the dynamics of alternate stable states at savanna—forest boundaries under frequent fire in central Brazil. Ecology 90:1326–1337
Holzschuh A, Steffan-Dewenter IS, Kleijn D, Tscharntke T (2007) Diversity of flower-visiting bees in cereal fields: effects of farming system, landscape composition and regional context. J Appl Ecol 44:41–49
Johnson SD, Steiner KE (2000) Generalization versus specialization in plant pollination systems. TREE 15:140–143
Kevan PG (1983) Floral colors through the insects eye: what they are and what they mean. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold, New York, pp 3–49
Kwaiser KS, Hendrix SD (2008) Diversity and abundance of bees (Hymenoptera: Apiformes) in native and ruderal grasslands of agriculturally dominated landscapes. Agr Ecosyst Environ 124:200–204
Larsson M, Franzen M (2007) Critical resource levels of pollen for the declining bee Andrena hattorfiana (Hymenoptera: Andrenidae). Biol Conserv 134:405–414
Lázaro A, Totland O (2014) The influence of floral symmetry, dependence on pollinators and pollination generalization on flower size variation. Ann Bot 114:157–165
Libano AM, Felfili JM (2006) Mudanças temporais na composicão florıstica e na diversidade de um cerrado sensu stricto do Brasil Central em um período de 18 anos (1985–2003). Acta Bot Bras 20:927–936
Machado IC, Lopes AV (2004) Floral traits and pollination systems in the Caatinga, a Brazilian tropical dry forest. Ann Bot 94:365–376
McCann KS (2000) The diversity-stability debate. Nature 405:228–233
Meirelles ML, Klink CA, Silva JCS (1997) Um modelo de estado y transiciones para el cerrado brasileño. Ecotropicos 10:45–50
Mendonça RC, Felfili JM, Walter BMT, Silva-Jr MC, Rezende AV, Filgueiras TS, Nogueira PE, Fagg CW (2008) Flora Vascular do bioma Cerrado: checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Planaltina, Embrapa Cerrados, pp 421–1279
Miranda HS, Sato MN, Nascimento-Neto R, Aires FS (2009) Fires in the cerrado, the Brazilian savanna. In: Cochrane MA (ed) Tropical fire ecology: climate change, land use, and ecosystem dynamics. Springer, Chichester, pp 427–450
Moreira AG (2000) Effects of fire protection on savanna structure in Central Brazil. J Biogeogr 27:1021–1029
Munhoz CBR, Felfili JM (2007) Reproductive phenology of an herbaceous subshrub layer of a Savannah (Campo Sujo) in the Cerrado Biosphere Reserve I, Brazil. Braz J Biol 67:299–307
Oliveira PE, Gibbs PE (2000) Reproductive biology of woody plants in a cerrado community of Central Brazil. Flora 195:311–329
Oliveira PE, Moreira AG (1992) Anemocoria em espécies de cerrado e mata de galeria de Brasília-DF. Rev Bras Bot 15:163–174
Oliveira PE, Paula FR (2001) Fenologia e biologia reprodutiva de plantas de Matas de Galeria. In: Ribeiro JF, Fonseca CE, Silva JC (eds) Cerrado: caracterização e recuperação de matas de galeria. Planaltina, Brasília, pp 303–332
Oliveira PE, Gibbs PE, Barbosa AA (2004) Moth pollination of woody species in the Cerrados of Central Brazil: a case of so much owed to so few? Plant Syst Evol 245:41–54
Oliveira Filho AT, Ratter JA (2002) Vegetation physiognomies and woody flora o the cerrado biome. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 91–120
Parrish JAD, Bazzaz FA (1979) Difference in pollination niche relationships in early and late successional plant communities. Ecology 60:597–610
Pinheiro F, Durigan G (2009) Dinâmica espaço-temporal (1962-2006) das fitofisionomias em unidade de conservação do Cerrado no sudeste do Brasil. Braz J Bot 32:441–454
Pinheiro F, Ribeiro JF (2001) Síndromes de dispersão de sementes em Matas de Galeria do Distrito Federal. In: Ribeiro JF, Fonseca CE, Silva JC (eds) Cerrado: caracterização e recuperação de matas de galeria. Planaltina, Brasília, pp 335–375
Pivello VR, Coutinho LM (1996) A quantitative successional model to assist in the management of Brazilian Cerrados. For Ecol Manag 87:127–138
Potts SG, Vulliamy B, Dafni A, Ne’eman G, Willmer P (2003a) Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84:2628–2642
Potts SG, Vulliamy B, Dafni A, Ne’eman G, O’Toole C, Roberts S, Willmer P (2003b) Response of plant–pollinator communities to fire: changes in diversity, abundance and floral reward structure. Oikos 101:103–112
Potts SG, Vulliamy B, Roberts S, O’Toole C, Dafni A, Ne’eman G, Willmer P (2004) Nectar resource diversity organises flower-visitor community structure. Entomol Exp Appl 113:103–107
Potts SG, Petanidou T, Roberts S, O’Toole C, Hulbert A, Willmer P (2006) Plant-pollinator biodiversity and pollination services in a complex Mediterranean landscape. Biol Conserv 129:519–529
Puyravaud JP, Pascal JP, Dufour C (1994) Ecotone structure as an indicator of changing forest-savanna boundaries (Linganamakki Region, Southern India). J Biogeogr 21:581–593
Ratter JA, Leitão Filho HF, Argent G, Gibbs PE, Semir J, Shepherd GJ, Tamashiro JY (1988) Floristic composition and community structure of a southern cerrado area in Brazil. Notes R Bot Gard Edinb 45:137–151
Roitman I, Felfili JM, Rezende AV (2008) Tree dynamics of a fire-protected cerrados sensu stricto surrounded by forest plantations, over a 13-year period (1991–2004) in Bahia, Brazil. Plant Ecol 197:255–267
Rosa R, Lima SC, Assunção LW (1991) Abordagem preliminar das condições climáticas de Uberlândia (MG). Sociedade e Natureza 3:91–108
Russell-Smith J, Edwards AC (2006) Seasonality and fire severity in savanna landscapes of monsoonal northern Australia. Int J Wildland Fire 15:307–317
Sato MN (2003) Efeito de longo prazo de queimadas prescritas na estrutura da comunidade lenhosa da vegetação do cerrado sensu stricto. Dissertation, Universidade de Brasília, Brasília
Schiavini I, Araújo GM (1989) Considerações sobre a vegetação da Reserva Ecológica do Panga (Uberlândia-MG). Sociedade e Natureza (Uberlandia) 1:61–66
Silberbauer-Gottsberger I, Eiten G (1983) Fitossociologia de um hectare de cerrado. Brasil Florest 54:55–70
Silberbauer-Gottsberger I, Gottsberger GA (1988) A polinização e plantas do cerrado. Rev Bras Biol 48:651–663
Silva WR, Sazima M (1995) Hawkmoth pollination in Cereus peruvianus, a columnar cactus of southeastern Brazil. Flora 190:339–343
Silva JA, Salomão AN, Gripp A, Leite EJ (1997) Phytosociological survey in Brazilian forest genetic reserve of Caçador. Plant Ecol 133:1–11
Soares JJ, Souza MHAO, Lima MIS (2006) Twenty years of post-fire plant succession in a “cerrado”, São Carlos, SP, Brazil. Braz J Biol 66:587–602
Steffan-Dewenter I, Tscharntke T (2001) Succession of bee communities on fallows. Ecography 24:83–93
Van Nuland ME, Haag EN, Bryant JA, Read QD, Klein RN, Douglas MJ, Gorman CE, Greenwell TD, Busby MW, Collins J, LeRoy JT, Schuchmann G, Schweitzer JA, Bailey JK (2013) Fire promotes pollinator visitation: implications for ameliorating declines of pollination services. PLoS One 8:e79853
Wardle DA, Jonsson M (2014) Long-term resilience of above-and belowground ecosystem components among contrasting ecosystems. Ecology 95:1836–1849
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. PNAS 96:1463–1468
Zamora R (2000) Functional equivalence in plant-animal interactions: ecological and evolutionary consequences. Oikos 88:442–447
Acknowledgments
We thank Felipe Amorim and Pietro Maruyama for their critical reading and commenting on the manuscript. We thank the three anonymous reviewers for comments and suggestions, which improved the manuscript. We thank the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Deus, F.F., Oliveira, P.E. Changes in floristic composition and pollination systems in a “Cerrado” community after 20 years of fire suppression. Braz. J. Bot 39, 1051–1063 (2016). https://doi.org/10.1007/s40415-016-0304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0304-9