Abstract
Purpose
To evaluate the efficacy and safety of monthly ranibizumab 0.5 mg in Chinese patients with subfoveal choroidal neovascularization (CNV) secondary to neovascular age-related macular degeneration (nAMD).
Methods
A 12-month open-label single-arm multicenter phase III study that included treatment-naïve (study eye) patients with primary/recurrent subfoveal CNV secondary to AMD. Patients (N = 114) aged ≥50 years with best-corrected visual acuity (BCVA) of 73–24 letters were treated with monthly ranibizumab for 12 months. Main outcomes were mean BCVA change from baseline to month 4 (primary endpoint) and over time to month 12, effects of ranibizumab treatment on retinal structure (months 4 and 12), and safety.
Results
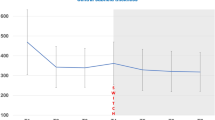
Ranibizumab led to significant improvements in mean BCVA ± standard error (SE) at both months 4 and 12 versus baseline (+9.5 ± 1.10 letters, 95 % confidence interval [CI] 7.3–11.7, and +12.7 ± 1.14 letters, 95 % CI 10.4–14.9, respectively, both P < 0.0001). Ranibizumab prevented loss of vision (≥0 letters BCVA gain) in 91.2 % of patients. Mean central retinal thickness ± SE reduced from baseline to month 12 (−119.9 ± 12.97 µm, 95 % CI −145.59 to −94.20, P < 0.0001). No new safety findings were reported in this study.
Conclusion
Ranibizumab administered monthly over 12 months was effective in improving BCVA and was well-tolerated in Chinese nAMD patients.





Similar content being viewed by others
References
Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51.
Hsu W-M, Cheng C-Y, Liu J-H, et al. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan- the Shihpai eye study. Ophthalmology. 2004;111(1):62–9.
Klein R, Klein BEK, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 radical/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–80.
Wong TY, Loon S-C, Saw S-M. The epidemiology of age related eye diseases in Asia. Br J Ophthalmol. 2006;90(4):506–11.
Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97.
Barbazetto IA, Takahashi BS. Verteporfin photodynamic therapy in the age of antiangiogenic therapy. Expert Rev Ophthalmol. 2008;3(4):365–83.
Ferrara N, Mass RD, Campa C, et al. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504.
Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–70.
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31.
Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239–48.
Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.
Boyer DS, Heier JS, Brown DM, et al. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731–9.
Schmidt-Erfurth U. Clinical safety of ranibizumab in age-related macular degeneration. Expert Opin Drug Saf. 2010;9(1):149–65.
Tano Y, Ohji M. EXTEND-I: safety and efficacy of ranibizumab in Japanese patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Acta Ophthalmol. 2010;88(3):309–316.
Kwon O-W, Lee FL, Chung H, et al. EXTEND III: efficacy and safety of ranibizumab in South Korean and Taiwanese patients with subfoveal CNV secondary to AMD. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1467–76.
Ferris FL III, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: Guidelines from the Eye Care technology Forum. Ophthalmology. 1996;103(1):181–2.
Ferris FL III, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–6.
Ferris FL III, Sperduto RD. Standardized illumination for visual acuity testing in clinical research. Am J Ophthalmol. 1982;94(1):97–8.
Chamberlin JA, Bressler NM, Bressler SB, et al. The use of fundus photographs and fluorescein angiograms in the identification and treatment of choroidal neovascularization in the macular photocoagulation study. Ophthalmology. 1989;96(10):1526–34.
Arevalo JF, Garcia RA. Clinical applications of optical coherence tomography in age-related macular degeneration. In: Arevalo JF, editor. Retinal angiography and optical coherence tomography. New York: Springer; 2009. p. 253–65.
Martin DF, Maguire MG, Ying GS, CATT Research Group, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908.
Kaiser PK, Boyer DS, Cruess AF, Denali Study Group, et al. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month results of the DENALI study. Ophthalmology. 2012;119(5):1001–10.
Yamashiro K, Tomita K, Tsujikawa A, et al. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol. 2012;154(1):125–36.
Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144(6):850–7.
Acknowledgments
A list of study team members for each of the seven medical centers appears in Appendix 1. The coauthors from each medical center contributed equally to the work. The authors thank Srividdya Agoram (for medical writing support), Ruchika Srinivasan (for her contribution towards various drafts of this manuscript), and Mayuri Shinde (for her editorial contribution) from Medical Communications, Novartis Healthcare Pvt. Ltd., India. Writing and editorial support was funded by Novartis.
Disclosure
The results from this study were presented in part at APAO 2012 and EURETINA 2012. Jialiang Zhao, Xiaoxin Li, Shibo Tang, Gezhi Xu, Xun Xu, Feng Zhang, and Meixia Zhang have no financial disclosures to make. Stefan Pilz, Jila Shamsazar, and Annette Nieweg are full-time employees of Novartis Pharma AG, Basel, Switzerland.
Funding
Novartis Pharma AG, Switzerland sponsored the study and was involved in the study conception and design, protocol writing, study drug provision, study coordination, data collection, data analysis, and interpretation. At all stages, the authors have had control over the content of this manuscript, for which they have given final approval and take full responsibility. Novartis Pharma AG enforces a ‘no ghost-writing’ policy. As funding sponsors, they have had the opportunity to review the manuscript but do not have authority to change any aspect of the manuscript. All authors (Jialiang Zhao, Xiaoxin Li, Shibo Tang, Gezhi Xu, Xun Xu, Feng Zhang, Meixia Zhang, Jila Shamsazar, Stefan Pilz, and Annette Nieweg) contributed to the conception, design, and conduct of the study, data collection, analysis, and interpretation; critical revision and final approval of the article.
Conflicts of interest
Stefan Pilz, Jila Shamsazar, and Annette Nieweg are full-time employees of Novartis Pharma AG, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Appendix 1
List of principal investigators and other important participants
Prof. Jialiang Zhao, Dr Junjie Ye, Dr Weiwei Wang, Dr Qi Zhou, Dr Ting Zhang, Ms Yajun Chen, Dr Ying YU, Dr Xiao Zhang, Prof. Fangtian Dong, Dr Shuran Wang, Prof. Youxin Chen, Ms Xiaoli Liu (Peking Union Medical College Hospital, Beijing China.)
Dr Xiaoxin Li, Dr Mingwei Zhao, Dr Yan Li, Dr Tong Qian, Ms Bin Liu, Dr Hong Yin, Dr Yi Chen, Ms Hongyan Li, Dr Huijun Qi (People's Hospital, Peking University, Beijing China.)
Prof. Feng Zhang, Dr Ying Xiong, Dr Liqin Gao, Dr Xuan Jiao, Dr Yu Mao (Beijing Tongren Hospital, Beijing China.)
Prof. Gezhi Xu, Prof. Qing Chang, Dr Lei Li, Dr Jia Yu, Dr Ling Wang, Prof. Yongjing Zhang, Dr. Wei Liu, Dr Rui Jiang (Eye Ear Nose and Throat Hospital of Fudan University, Shanghai China.)
Prof. Xun Xu, Dr Weiran Niu, Associate Prof. Bilian Ke, Ms Ping Zhu, Dr Weijun Wang, Ms Qian Li (Shanghai 1st People's Hospital, Jiao Tong University, Shanghai China.)
Prof. Shibo Tang, Mr Hongxing Diao, Ms Meifeng He, Dr Honghua Yu, Ms Jianying Pan, Dr Jie Hu, Dr Qingyun Liu, Dr Xiaobo Zhu, Mr Guohong Feng, Ms Shaofen Lin, Dr Xiaoyan Ding, Dr Andina Hu, Dr Wei Ma, Dr Xuting Hu, Dr Tao Li (Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou China.)
Associate Prof. Meixia Zhang, Prof. Junjun Zhang, Dr Bi Yang, Ms Ronghai Wang, Dr Fang Lu, Dr Ming Zou (West China Hospital of Sichuan University, Chengdu Sichuan China.)
Rights and permissions
About this article
Cite this article
Zhao, J., Li, X., Tang, S. et al. EXTEND II: An Open-Label Phase III Multicentre Study to Evaluate Efficacy and Safety of Ranibizumab in Chinese Patients with Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. BioDrugs 28, 527–536 (2014). https://doi.org/10.1007/s40259-014-0106-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-014-0106-1