Abstract
Purpose of Review
To review the topic of osseointegration amputation reconstruction, which inserts a transcutaneous metal implant into the remaining intramedullary bone of a person with an amputation to facilitate a direct bone-anchored connection to an external prosthesis, eliminating the molded socket interface.
Recent Findings
Evidence continues to build that patients function better and have a higher quality of life with osseointegration implants compared with traditional socket prosthetics. The indications for osseointegration are expanding to additional patient populations and the long-term outcomes available are favorable which supports the continued refinement and utilization of the technology.
Summary
Osseointegration implants offer people with amputations freedom from burdensome socket prosthetics while improving function and quality of life. Mild infections at the skin interface are common but managed effectively with oral antibiotics and rarely lead to deep infection and implant removal. Other serious complications like hip or implant fracture are also uncommon. Additional long-term outcomes are needed along with technologic refinements, especially at the skin implant interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
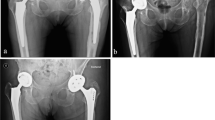
Historically, the treatment and rehabilitation of individuals with upper or lower extremity amputations utilized socket-mounted prostheses. Technologic innovations to socket materials and liners, including silicone interfaces and light weight custom-molded carbon fiber shells have made sockets easier to wear for many people with amputations. However, many continue to experience significant difficulties, specifically with the socket-residuum interface, prompting frequent trips to the prosthetist to optimize fit and comfort [1]. Sweating, pinching, skin breakdown, poor fit, and timely donning and doffing are all issues leading to a lack of prosthetic use and diminished quality of life among people with amputations [2]. The most effective method for eliminating these problems is to eliminate the socket entirely with an osseointegration implant. These metal implants are directly anchored to the residual bone via the intramedullary canal and a prosthetic limb is attached to their extracorporeal metal abutment (Figure 1). The bone grows onto the surface of the implant, creating a stable and durable interface.
A Standing radiograph of a transtibial osseointegration amputation reconstruction. B Picture of the patient’s stoma. Patient required amputation following a motorcycle collision. Underwent osseointegration implantation 1 year later to improve function. Osseointegrated Prosthetic Limb (OPL) system utilized
After years of experimentation in the 1950s, osseointegration was successfully employed in humans by Dr. Per-Ingvar Branemark in 1965 when he inserted titanium screws into the jaw to aid a man with no teeth [3]. His son, Rickard Branemark, was an orthopedic surgeon and modified the technology for use in the limbs in the 1990s [4]. Now osseointegration has been used in Europe for over 25 years and continues to expand worldwide [5, 6]. The indications for the procedure continue to expand as well. Initially implanted in individuals with a non-vascular transfemoral amputation, more recently osseointegration reconstruction has been utilized in the tibia, upper extremities, and in individuals with stable vascular disease [7].
The benefits of osseointegration beyond relieving socket-related issues include unimpeded joint range of motion, increased prosthetic wear time, osseoperception (the mechanical stimulation transduced by mechanoreceptors in bone that inform the central nervous system of positional awareness [8]), and reduced oxygen consumption with activity [9]. These changes are associated with improved mobility and quality of life along with a renewed connection with the limb [10]. With the ongoing utilization of bone-anchored osseointegration prostheses, immediate reconstruction is now considered at the time of primary amputation. Concerns regarding infection remain and will be discussed in detail [11, 12]. Full Food and Drug Administration (FDA) approval is still pending for most implants which limits widespread adoption of the technology in the USA, but this is expected to be reevaluated in the coming years.
Patient Evaluation
Following a typical amputation, the incisions are allowed to heal and the limb is compressed of its edema for several weeks. The patient can then be fitted for a socket prosthesis, which often starts with adjustable straps to accommodate an evolving limb size. The next socket fabricated will then utilize custom molds to facilitate a well-fitted socket. The process usually takes months and may include multiple liner or socket revisions before a useful prosthesis is attained. At higher amputation levels, the socket prosthetic becomes even more challenging to fit and often results in discomfort, local pain, pinching, and skin ulceration [13, 14]. Skin grafts, scarring, and heterotopic bone may preclude the use of a socket prosthesis entirely. Even in the best case, the prosthesis typically hinders the range of motion of the adjacent joint. As patients age, the size and shape of the residual limb changes further, often necessitating a new socket, which becomes quite costly [15]. A portion of people with amputations will be satisfied with their socket prosthetic [16]; however, many people with amputations experience symptomatic socket-residuum interface problems leading to reduced prosthetic use and markedly reduced quality of life [2, 13, 14].
The original indication for an osseointegration implant was a patient with an above-knee amputation with decreased ambulation due to difficulty tolerating a socket prosthetic [4]. The objective of osseointegration was to utilize the remaining bone and liberate the deficient soft tissue from the role of locomotion. This remains the most common indication, and encompasses amputations performed due to trauma, infection, neoplasm, or vascular disease. While patients with below-knee amputations often have fewer problems with a socket prosthesis, transmitting force from the ground to the skeleton through a socket and soft tissue envelope remains inefficient [9], so high demand patients (e.g. laborers) are also indicated for osseointegration. Other indications for a person with a below-knee amputation to pursue osseointegration include peroneal and tibial neuromas, compromised soft tissue from infection or trauma, or a short residual tibia not amenable to a socket. In the upper extremity, maintaining range of motion of the elbow and shoulder is essential to tasks of daily living, so bulky socket prostheses which hinder motion are often discarded [17]. Osseointegration implants in the ulna, radius or humerus can support the weight of a hand and wrist prosthetic, eliminating the cumbersome straps or harnesses which limit elbow and shoulder range of motion [18] [19]. They can also be utilized with myoelectric control of a robotic hand and wrist, further improving upper extremity function [20].
To determine if a patient is eligible for osseointegration, a physical examination is performed to ensure that adequate muscular strength is present to power the residual joints. Wheelchair use for mobility patients with otherwise intact strength and joint motion often regains the ability to ambulate after implantation, so is not considered a contraindication to surgery [21]. Orthogonal calibrated radiographs of the involved bone are obtained to determine if bone resection is necessary, the available length for the intramedullary canal, and if other abnormalities in the bone or soft tissue must be addressed. Given the relative infancy of the procedure and the current reliance on custom implants, computerized tomography (CT) scans are used in all cases to plan the 3D geometry of the implant. The diameter of the implant is determined using the endosteal bone diameter at multiple levels along the bone. The implant must fit the bone snugly for osseointegration to be successful and to prevent early loosening. In the future, off-the-shelf sizing will ideally be available, similar to hip and knee arthroplasty. If there is any concern for residual infection from a previous traumatic amputation, screening blood tests (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) are obtained. Elevation in ESR or CRP or any abnormalities on radiographs or CT warrant further investigation with magnetic resonance imaging (MRI). Infection should be eradicated prior to implantation. Whether this requires sterilization of the canal with an antibiotic spacer for a time is controversial and likely depends on the severity of the residual infection.
Although still being defined and evaluated, contraindications for surgery generally include an extremely short bone segment (< 4 or 5 cm), history of radiation directly to the bone, active chemotherapy, progressive peripheral vascular disease, active smoking, uncontrolled diabetes, and morbid obesity exceeding the weight threshold of the implant (varies by implant). The patient must also be willing to undergo substantial rehabilitation following the operation to reach their full potential [22]. As the procedure evolves, the indications for surgery will likely broaden, the contraindications better defined, and the optimal rehabilitation protocols disseminated.
Implant Design and Surgical Details
Two predominant implant designs are currently in use—screw-type and press-fit. They have significant differences regarding surgical technique, rehabilitation, and time to ambulation but ultimately rely on the same principle—the creation of a bone and metal interface that is tightly opposed at the microscopic level. The bone metal interface is similar to that of an uncemented femoral stem in a hip replacement and likewise stable enough to support weight-bearing activity.
The Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA, Integrum AB, Sweden) is the oldest osseointegration implant and was used in the first osseointegration procedure pioneered by Brånemark in Sweden in 1990 (Table 1) [4]. Its outer threads screw into the bone like the original technique used in the jaw. The length of the intramedullary portion of the OPRA implant is 80 mm. Using the standard OPRA technique, two surgical procedures are performed 6 months apart to allow adequate implant integration with the host bone [23]. The first procedure implants the threaded intramedullary bone anchor and the distal soft tissue is fully closed. The second procedure creates a stoma at the skin-implant interface and attaches the transcutaneous abutment to the implanted fixture. A short training prosthesis is then attached and the patient increases load on the bone until a full leg prosthesis is attached at around 6 weeks. At 4 months, the patients are encouraged to increase prosthetic wear time and at 6 months graduate to independent walking without crutches if possible. Over 500 OPRAs have been implanted according to the company website. It is approved for use in the femur by the US FDA.
The Integral Lep Prosthesis (ILP, previously Endo-Exo Prosthesis, Eska Orthopaedics GmbH, Germany) implant was developed in Germany by Hans Grundei. The design is 140–180 mm in length, significantly longer than the OPRA [24]. The intramedullary canal is prepared via sequential reaming and broaching until a press-fit of the implant with the bone is obtained and a temporary plug is inserted into the distal end of the implant. Approximately 6 weeks later, a circular coring blade is used to open the skin over the abutment to create a stoma. The implant plug is removed, and a dual cone adapter is inserted percutaneously. Rehabilitation proceeds as tolerated and the permanent prosthetic limb is attached within the first few weeks after the second stage. The change in latency from implantation to stoma creation from 6 months to 6 weeks means the prosthetic leg is attached more quickly than with the OPRA. The implant is widely used in Europe but not in the USA.
The first implant utilized for single-stage implantation (i.e. immediate stoma creation) was the Osseointegrated Prosthetic Limb (OPL, Osseointegration International/Permedica SpA, Italy), designed by Al Muderis (Fig. 2) [6]. Also, a press-fit implant, it employs a titanium stem with a macroporous surface coating that facilitates bone ingrowth. The typical length is 140 mm in the femur, similar to the ILP. In patients with adequate bone, weight-bearing exercises often start immediately after surgery. Al Muderis and his group have implanted over 750 OPLs in the last several years in the femur and tibia. To use this implant in the USA, a humanitarian device exemption needs to be filed with the FDA prior to surgery. A similar system, the Osseointegrated Femur and Tibia (OTN Implant BV, the Netherlands), was developed and is in use in the Netherlands and other parts of Europe. Other implants with very early results include the Percutaneous Osseointegrated Prosthesis (DJO Orthopedics, Austin, TX, USA) under clinical trial [25] and the Compress device (Zimmer Biomet, Warsaw, IN, USA), primarily used for tumor endoprosthetic reconstruction but modified with a transcutaneous prosthetic adaptor (Table 1) [26].
Once the implant is inserted in the bone, the muscle, subcutaneous tissue, and skin must be closed around the transcutaneous portion. There is less published literature on the details of this topic, so methods vary [27]. Some surgeons support opposing the bone end as close to the skin as possible without intervening muscle so that the skin adheres to the bone like an antler. Others perform a purse string myoplasty over the bone end and the subcutaneous fat is thinned but the skin maintains a robust vascular supply and can grow around the implant. The skin is more mobile but over time forms a loose seal around the prosthesis. Regardless, once the implant is osseointegrated, the bone/implant interface is seemingly resistant to deep infection, discussed further below, which has allowed the technology to persist.
All implant designs today have smooth polished metal traversing the skin because textured designs lead to a high rate of pain, drainage, and ultimately stoma revisions and have been abandoned [28]. In general, excess soft tissue can impinge on the attached prosthesis or lead to pistoning, drainage, bleeding, inflammation, and increased shear on the skin (which may contribute to superficial infection) so care is taken to remove as much excess tissue as possible around the stoma (Fig. 3). A plastic surgeon can be helpful with this portion of the procedure, shaping the soft tissue in a smooth contour around the stoma, and ultimately decreasing soft tissue complications [27].
A Picture of a transfemoral osseointegration implant and stoma, noting soft tissue contouring around implant. Patient required amputation following a motor vehicle collision. Had difficulty utilizing a socket prosthetic due to complex scarring in residual limb and underwent osseointegration 3 years later. B Picture of patient standing with leg attached. Osseointegrated Prosthetic Limb (OPL) system utilized
Rehabilitation
There is no universal method for loading the prosthesis in the postoperative period. The decision should be patient-specific and based on the quality of bone encountered in the OR, the appearance of the bone and implant on XR/CT, the strength and balance of the patient, and the risk of falling while adapting to a new prosthesis. The screw type implants are gradually loaded immediately after the second surgery because the 3–6-month latency period allows for osseointegration. A short trainer prosthesis is utilized initially, with 20-kg loading performed for ~ 30 min twice a day and gradually increasing to full body weight at ~ 10 kg/wk [29]. Any pain rated greater than a 5 on the visual analog scale warrants an evaluation to ensure fracture has not occurred. Prosthetic gait training then begins around 12 weeks in the presence of a therapist. Walking is initiated with two crutches on flat surfaces for 1–2 h a day, then progressed to full prosthetic use in 4–6 weeks.
For press-fit implants contacting a long segment of diaphyseal bone with normal density, gradual loading can begin immediately following surgery with the goal to attach a prosthetic leg at six to 10 weeks. Reasons to restrict weight bearing to allow initial osseointegration include thin cortical bone, osteopenic bone, shorter implant contact length, a cortical crack encountered during implantation, and predominantly metaphyseal fit (common in the tibia). At the surgeon’s discretion, an initial period of rest without any loading may be advisable. Like the screw fit implants, the press-fit implant is loaded through a short trainer prosthesis, which limits the gravitational pull of the implant and early loosening or dislodgement. An example loading protocol applies 20 lbs for 10–15 min, 4–6 times per day, with a gradual increase of 5 lbs per day or every other day [5]. Any pain slows down or halts the progression and imaging is obtained. Once the patient can apply over half body weight through the prosthesis, a prosthetic leg can be attached (Fig. 4). Walking then is protected with crutches or a walker for the next 6 weeks, and gait training is initiated with a physical therapist. For patients with a transfemoral amputation, utilization of a cane or crutch for long-term ambulatory assistance is not considered a failure of treatment, while nearly all transtibial amputees will walk unaided [5].
Outcomes and Complications
Osseointegration has emerged over the past two decades as a dramatically different approach to limb reconstruction following amputation, overcoming myriad issues associated with traditional socket prosthesis. However, no procedure is without complications. The most common identified is soft tissue infection at the stoma not involving the underlying bone or implant. Stoma revisions due to excess tissue or irritation are also frequently reported. Less common but more significant complications include osteomyelitis, periprosthetic hip fractures, implant fractures, and implant loosening.
Patient-reported and functional outcome measures have guided whether freedom from a socket prosthesis outweighs the potential complications of osseointegration. The most common instrument in use is the Questionnaire for persons with a Transfemoral Amputation (QTFA), which was developed to measure the success of osseointegration implants versus socket prosthetics. Its domains include prosthetic use (hours per day), patient mobility, prosthetic problems, and overall experience as a person with an amputation.
Short-Term Outcomes
Early studies on osseointegration all focused on transfemoral amputations given the difficulty of wearing an ischial-bearing socket. The results in over 50 patients from the Swedish group where the procedure originated demonstrated that QTFA scores improved significantly across all domains [21, 23]. Four patients had their implants removed (one due to infection, three for loosening), and three sustained ipsilateral hip fractures. However, it is worth noting that most of the patients with failures requested reimplantation despite complications. Another early study from Germany reported that 35 out of 37 patients said they would have the procedure again [24] and there was only one deep infection and two hip fractures [28]. Another European group measured patients 1-year post-osseointegration and demonstrated significant improvements in the QTFA Prosthetic Use (56 h/wk to 101 h/wk) and Global Score (39 to 63) along with substantial improvement in the 6-min walk test (321 ft to 423 ft) and Timed Up and Go score (15.1 s to 8.1 s) [9]. They also demonstrated decreased oxygen consumption from 1330 mL/min to 1093 mL/min compared with a socket. The Australian group practicing osseointegration also demonstrated significantly improved outcomes in all QTFA domains in 50 above-knee amputations with an average 21.5-month follow-up [30]. Given this data, it is reasonable to conclude that osseointegration implants improve a patient’s experience with an amputation compared with socket prosthetics in the short term.
Long-Term Outcomes
Within the last several years, a few centers have published studies on the intermediate term outcomes of osseointegration in patients with transfemoral amputations, most notably in Sweden and other parts of Europe. The OPRA study between 1999 and 2007 followed 51 patients with 55 transfemoral amputations prospectively for 2 and 5 years. There were three patients who withdrew from the study for reasons unrelated to osseointegration, and three patients who had their implants removed. The aggregate survival rate was 92% at 2-year follow up, 40 out of 45 patients (89%) reported daily use of the prosthesis, compared with 57% before implantation. The mean prosthetic use score improved dramatically, as did the remainder of QTFA domains. The overall implant survival rate of this population remained at 92% at 5-year follow-up [21]. At 15 years, the longest follow-up published at this time, the patient-reported outcomes remained significantly better compared with their pretreatment scores. The survival rate of the osseointegrated portion (fixture) was 89% and 72% after seven and 15 years, respectively. Approximately 55% of these patients had to exchange the external abutment at least once which was associated with higher activity levels, but 64% of the patients stated that their overall situation as an amputee was better due to osseointegration, while one said it was worse [31].
Results from the UK with minimum 9-year follow-up by Matthews et al. had an implant retention rate of 80% once the OPRA protocol for rehabilitation was established (3 of 5 early implants before the protocol were removed). In total, five implants were explanted; three due to deep infection, one for chronic pain, and one due to implant fracture. The results from the QTFA showed significant improvements in quality of life up to 5 years following implantation along with improvement in the physical function and physical component score of the SF-36 [22]. A study by Ranker et al. from Germany with average 6.3-year follow-up demonstrated that 81% of patients had no complications, 7% had stoma problems, 6% sustained a periprosthetic fracture, and 3% were explanted due to infection [32]. These groups demonstrate that osseointegrated implants allow for prolonged prosthetic usage and improved quality of life with an acceptable risk of major complications as compared to traditional prostheses. Long-term outcomes in large groups of patients are lacking at this time but will be crucial for establishing survival and complication rates.
Complications
With a transcutaneous metal implant, there will always be a risk of infection with osseointegration. Colonization of the stoma region clearly occurs—Lenneras et al. demonstrated that 27 of 30 patients undergoing surgery for an abutment change were culture positive for a host of different bacteria, but only 3 underwent explantation [33]. Superficial infection, usually diagnosed via redness or drainage at the stoma, has been a commonly reported complication of the procedure in the short and long term, but prompt administration of antibiotics prevents most infections from progressing to a true periprosthetic infection requiring explantation. In the Matthews et al. study, 61% of the patients experienced occasional superficial stoma infections managed with oral antibiotics that did not interrupt prosthetic use [22]. A study of the OPRA population specifically addressing osteomyelitis found that the 10-year estimated risk of osteomyelitis was 20% with most diagnosed early, 2.6 years (median) from implantation [11]. Some of the infections were successfully treated with antibiotics and minor debridements, so the 10-year risk of explant due to infection was 9%. There were no patient factors associated with infection, including smoking or diabetes. Another study assessing infection risk in 91 implants with 3-year mean follow-up found 41 cases (28% of patients) of mild infection at the stoma which accounted for 87% of all infections. Swabs taken determined Staphlococcus aureus and coagulase-negative staphylococci to be the most common organisms. Two other infections required parenteral antibiotics, four required surgical debridement, but none lead to explantation [34]. The reason why certain colonizations remain dormant while others develop into osteomyelitis and septic loosening is not clear. But overall, the rates of deep infection leading to explant have remained less than 10% in most studies, and the clear functional gains exhibited outweigh the occasional stoma infections in the minds of surgeons and patients alike. However, it remains necessary to inform patients regarding the risk of infection prior to surgery.
Periprosthetic hip fracture is a notable complication that must be considered given its occurrence in any large series of osseointegration. In transfemoral reconstructions, the metal stem usually extends to the subtrochanteric region of the femur leaving the intertrochanteric and femoral neck at risk for fracture with falls or trauma. For perspective, it has been found that around 3% of patients with an amputation with socket-mounted prostheses fracture within 5 years [35]. Hoellwarth et al. performed a retrospective review of 518 osseointegration procedures and found a fracture rate of 4.2% overall and 6.3% when isolating femoral implants. Importantly, no implants needed to be removed, all fractures united, and no patients lost a functional level (using k-level system) after fracture healing. Female sex and increasing weight were risk factors for fracture, but interestingly bone density was not [36].
With longer use and follow-up of press-fit osseointegration systems, intraosseous implant fracture has also been identified as a potential complication. In a recent review of 58 press-fit osseointegration stems with minimum 5-year follow-up, six stems fractured, and smaller stem diameter < / = 15 mm was found to be a significant predictor of failure versus stems > / = 17 mm [37].
Recent Developments
As the procedure has gained acceptance, osseointegration implants are being utilized in other populations, including transtibial amputations, patients with vascular disease, and in patients with refractory complex regional pain syndrome. A recent series including 13 transtibial reconstructions demonstrated similar improvement across QTFA domains (also utilized for transtibial amputations) as well as PROMIS function, pain interference, and global mental and physical scores with average follow-up of almost 2 years [5]. Two explanations (one for infection, one for loosening) in the transtibial group were both successfully reimplanted. Peripheral vascular disease (PVD) is one of the leading causes of lower limb amputation [38] and studies have shown that quality of life is impacted by subsequent mobility impairment [9]. Patients with PVD who require transtibial amputation often have skin compromise which poses a significant challenge for a traditional socket prosthesis. A recent case series by Akhtar et al. found that six of six patients with PVD experienced improved mobility following osseointegration, including three that were wheelchair use for mobility previously [7]. If further reports demonstrate its safety in this population, it would expand the indication for the procedure substantially. Finally, a series by Hoellwarth et al. of three patients who underwent transfemoral amputation for complex regional pain syndrome demonstrated that all three were able to ambulate following surgery (two independent, one with crutches) [39]. The authors have also treated two patients for this indication and both are satisfied with their decision and ambulate independently.
Not surprisingly, there has also been interest in the remodeling of bone around the osseointegration implants over time. Nebergall et al. evaluated the screw type OPRA implant using radiostereometric analysis and found minimal migration at two and 5 years as evidenced by 0.02 mm distal migration and 0.42 degree rotational movement [40]. There was some early stress shielding similar to that seen with femoral stems at 2 years that decreased at 5 years. Orgel et al. evaluated the ILP/Endo-Exo Prosthesis for cortical changes on radiographs at 3 years and discovered both cortical hypertrophy and atrophy at similar rates (~ 40%) or both concurrently across different segments of the implant (20%), but no overall significant differences in cortical thickness [41]. Ultimately, quantitative CT densitometry measurements will be more sensitive to the changes that occur within the bone after osseointegration implantation, but given CT scans are rarely needed after implantation, this will require specific study.
Conclusion
Osseointegrated prostheses offer a rehabilitation option for patients with an amputation that provides increased mobility, higher satisfaction, and greater use than traditional socket prostheses. Despite skepticism regarding infection risk, osseointegration prostheses have demonstrated excellent survival and patient-reported outcomes in multiple studies with both early and long-term follow-up. There are currently several different osseointegrated implant designs, surgical techniques, and rehabilitation protocols, each with its strengths and limitations. The two most severe complications, deep infection and periprosthetic fracture, are infrequent, and the most common complication, stoma infection, is easily managed. The results to date have led a growing number of surgeons to add osseointegration to their armamentarium. In the USA, regulatory barriers remain for now, but further adoption of these implants should lead to more availability, collaboration, and refinement of the procedure. It is conceivable that one day osseointegrated implants will overtake socket prosthetics as the dominant form of amputation reconstruction.
References
Haggstrom EE, Hansson E, Hagberg K. Comparison of prosthetic costs and service between osseointegrated and conventional suspended transfemoral prostheses. Prosthet Orthot Int. 2013;37:152–60. https://doi.org/10.1177/0309364612454160.
Pezzin LE, Dillingham TR, Mackenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. 2004;85:723–9. https://doi.org/10.1016/j.apmr.2003.06.002.
Branemark P, Zarb G, Albrektsson T. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. 1st ed. Chicago: Quintessence Publiishing; 1985.
Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38:175–81.
Reif TJ, Khabyeh-Hasbani N, Jaime KM, Sheridan GA, Otterburn DM, Rozbruch SR. Early experience with femoral and tibial bone-anchored osseointegration prostheses. JBJS Open Access. 2021;6:e21.00072. https://doi.org/10.2106/JBJS.OA.21.00072.
Muderis MA, Lu W, Tetsworth K, Bosley B, Li JJ. Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: the Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study. BMJ Open. 2017;7:e013508. https://doi.org/10.1136/bmjopen-2016-013508.
Akhtar MA, Hoellwarth JS, Al-Jawazneh S, Lu W, Roberts C, Al Muderis M. Transtibial osseointegration for patients with peripheral vascular disease: a case series of 6 patients with minimum 3-year follow-up. JBJS Open Access. 2021;6:e20.00113. https://doi.org/10.2106/JBJS.OA.20.00113.
Jacobs R, Brånemark R, Olmarker K, Rydevik B, Van Steenberghe D, Brånemark PI. Evaluation of the psychophysical detection threshold level for vibrotactile and pressure stimulation of prosthetic limbs using bone anchorage or soft tissue support. Prosthet Orthot Int. 2000;24:133–42. https://doi.org/10.1080/03093640008726536.
Meent H, Hopman MT, Frölke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94:2174–8. https://doi.org/10.1016/j.apmr.2013.05.020.
Lundberg M, Hagberg K, Bullington J. My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. 2011;35:207–14. https://doi.org/10.1177/0309364611409795.
Tillander J, Hagberg K, Berlin Ö, Hagberg L, Brånemark R. Osteomyelitis risk in patients with transfemoral amputations treated with osseointegration prostheses. Clin Orthop Relat Res. 2017;475:3100–8. https://doi.org/10.1007/s11999-017-5507-2.
Atallah R, van de Meent H, Verhamme L, Frölke JP, Leijendekkers RA. Safety, prosthesis wearing time and health-related quality of life of lower extremity bone-anchored prostheses using a press-fit titanium osseointegration implant: a prospective one-year follow-up cohort study. PLoS One. 2020;15:e0230027. https://doi.org/10.1371/journal.pone.0230027.
Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186–94. https://doi.org/10.1080/03093640108726601.
Sullivan J, Uden M, Robinson KP, Sooriakumaran S. Rehabilitation of the trans-femoral amputee with an osseointegrated prosthesis: the United Kingdom experience. Prosthet Orthot Int. 2003;27:114–20. https://doi.org/10.1080/03093640308726667.
Black GG, Wu X, Rozbruch SR, Otterburn DM. A solution to poorly tolerated lower limb amputations: osseointegrated prostheses prove cost-effective in the United States. Plast Reconstr Surg – Glob Open. 2021;9:124. https://doi.org/10.1097/01.GOX.0000799736.63628.5f.
Gholizadeh H, Abu Osman NA, Eshraghi A, Ali S, Yahyavi ES. Satisfaction and problems experienced with transfemoral suspension systems: a comparison between common suction socket and seal-in liner. Arch Phys Med Rehabil. 2013;94:1584–9. https://doi.org/10.1016/j.apmr.2012.12.007.
Pierrie SN, Gaston RG, Loeffler BJ. Current concepts in upper-extremity amputation. J Hand Surg Am. 2018;43:657–67. https://doi.org/10.1016/j.jhsa.2018.03.053.
Jönsson S, Caine-Winterberger K, Brånemark R. Osseointegration amputation prostheses on the upper limbs: methods, prosthetics and rehabilitation. Prosthet Orthot Int. 2011;35:190–200. https://doi.org/10.1177/0309364611409003.
Tsikandylakis G, Berlin Ö, Brånemark R. Implant survival, adverse events, and bone remodeling of osseointegrated percutaneous implants for transhumeral amputees. Clin Orthop Relat Res. 2014;472:2947–56. https://doi.org/10.1007/s11999-014-3695-6.
Jaime KM, Reif TJ, Kafedzic H, Garrison G, Dhawan J, Rozbruch SR. Attachment of a myoelectric prosthesis after transulnar osseointegration implantation: a 2-patient case study. JBJS Case Connect. 2021;11; https://doi.org/10.2106/JBJS.CC.21.00381
Brånemark RP, Hagberg K, Kulbacka-Ortiz K, Berlin Ö, Rydevik B. Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications. J Am Acad Orthop Surg. 2019;27:e743-51. https://doi.org/10.5435/JAAOS-D-17-00621.
Matthews DJ, Arastu M, Uden M, Sullivan JP, Bolsakova K, Robinson K, Sooriakumaran S, Ward D. UK trial of the osseointegrated prosthesis for the rehabilitation for amputees: 1995–2018. Prosthet Orthot Int. 2019;43:112–22. https://doi.org/10.1177/0309364618791616.
Brånemark R, Berlin Ö, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation. Bone Joint J. 2014;96-B:106–13. https://doi.org/10.1302/0301-620X.96B1.31905.
Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. J Bone Joint Surg Am. 2010;92(Suppl 2):180–6. https://doi.org/10.2106/JBJS.J.00806.
Agarwal J, Kubiak E, Gililland J, Gillespie B, Peter Beck J, Sinclair S. Abstract development of a percutaneous prosthesis for transfemoral amputees the Utah experience. Plast Reconstr Surg Glob Open. 2018;6:95–6.
McGough RL, Goodman MA, Randall RL, Forsberg JA, Potter BK, Lindsey B. The Compress® transcutaneous implant for rehabilitation following limb amputation. Unfallchirurg. 2017;120:300–5. https://doi.org/10.1007/s00113-017-0339-9.
Marano AA, Modiri O, Rozbruch SR, Otterburn DM. Soft tissue contouring at the time of osseointegrated implant reconstruction for lower extremity amputation. Ann Plast Surg. 2020;85:S33-6. https://doi.org/10.1097/SAP.0000000000002329.
Juhnke D, Beck JP, Jeyapalina S, Aschoff HH. Fifteen years of experience with integral-leg-prosthesis: cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52:407–20. https://doi.org/10.1682/JRRD.2014.11.0280.
Hagberg K, Brånemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses—rehabilitation perspective. J Rehabil Res Dev. 2009;46:331–44.
Muderis MA, Tetsworth K, Khemka A, Wilmot S, Bosley B, Lord SJ, Glatt V. The Osseointegration Group of Australia Accelerated Protocol (OGAAP-1) for two-stage osseointegrated reconstruction of amputated limbs. Bone Joint J. 2016;98-B:952–60. https://doi.org/10.1302/0301-620X.98B7.37547.
Hagberg K, GhassemiJahani S, Kulbacka-Ortiz K, Thomsen P, Malchau H, Reinholdt C. A 15-year follow-up of transfemoral amputees with bone-anchored transcutaneous prostheses: mechanical complications and patient-reported outcomes. Bone Joint J. 2020;102 B:55–63. https://doi.org/10.1302/0301-620X.102B1.BJJ-2019-0611.R1.
Ranker A, Örgel M, Beck JP, Krettek C, Aschoff HH. [Transcutaneous Osseointegrated Prosthetic Systems (TOPS) for Transfemoral Amputees - A Six-Year Retrospective Analysis of the Latest Prosthetic Design in Germany]. Rehabil (Stuttg). 2020;59:357–65. https://doi.org/10.1055/a-1223-3205.
Lennerås M, Tsikandylakis G, Trobos M, Omar O, Vazirisani F, Palmquist A, Berlin Ö, Brånemark R, Thomsen P. The clinical, radiological, microbiological, and molecular profile of the skin-penetration site of transfemoral amputees treated with bone-anchored prostheses. J Biomed Mater Res A. 2017;105:578–89. https://doi.org/10.1002/jbm.a.35935.
Muderis MA, Khemka A, Lord S, Meent HVd, Frölke JP. Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study. J Bone Joint Surg. 2016;98:900–9. https://doi.org/10.2106/JBJS.15.00808.
Hoellwarth JS, Tetsworth K, Rozbruch SR, Handal MB. A Coughlan, M Al Muderis 2020 Osseointegration for amputees: current implants, techniques, and future directions. JBJS Rev. 2020;8:e0043. https://doi.org/10.2106/JBJS.RVW.19.00043.
Hoellwarth JS, Tetsworth K, Kendrew J, Kang NV, Waes van O, Al-Maawi Q, Roberts C, Al Muderis M. Periprosthetic osseointegration fractures are infrequent and management is familiar. Bone Joint J. 2020;102-B:162–9. https://doi.org/10.1302/0301-620X.102B2.BJJ-2019-0697.R2.
Mohamed J, Reetz D, van de Meent H, Schreuder H, Frölke JP, Leijendekkers R. What are the risk factors for mechanical failure and loosening of a transfemoral osseointegrated implant system in patients with a lower-limb amputation? Clin Orthop Relat Res. 2021. https://doi.org/10.1097/CORR.0000000000002074.
Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Phys Med Rehabil Clin N Am. 2014;25:1–8. https://doi.org/10.1016/j.pmr.2013.09.001.
Hoellwarth JS, Al-Jawazneh SS, Tetsworth K, Lu W, Roberts C, Al Muderis M. Amputation with osseointegration for patients with intractable complex regional pain syndrome: a report of 3 cases. JBJS Case Connect. 2021;11:e20.00267. https://doi.org/10.2106/JBJS.CC.20.00267.
Nebergall A, Bragdon C, Antonellis A, Kärrholm J, Brånemark R, Malchau H. Stable fixation of an osseointegated implant system for above-the-knee amputees: titel RSA and radiographic evaluation of migration and bone remodeling in 55 cases. Acta Orthop. 2012;83:121–8. https://doi.org/10.3109/17453674.2012.678799.
Örgel M, Liodakis E, Jaratjitwilai P, Harb A, Wirries N, Omar M, Krettek C, Aschoff H. Three-year follow-up of changes of cortical bone thickness after implantation of Endo-Exo-Prosthesis (EEP) for transfemoral amputees. J Orthop Surg Res. 2020;15:164. https://doi.org/10.1186/s13018-020-01675-w.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
All reported studies with human subjects have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
Authors Reif and Jacobs declare that he/she has no conflict of interest.
Author Fragomen has received consulting fees from implant companies Nuvasive, Smith & Nephew, and Depuy Synthes.
Author Rozbruch has received royalties and consulting fees from implant company Nuvasive and stock in implant company Orthospin.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Amputation Rehabilitation
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reif, T.J., Jacobs, D., Fragomen, A.T. et al. Osseointegration Amputation Reconstruction. Curr Phys Med Rehabil Rep 10, 61–70 (2022). https://doi.org/10.1007/s40141-022-00344-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-022-00344-9