Abstract
Traumatic brain injury is a dynamic condition with varying severities, classifications, and periods. Treatments and interventions through these periods can dramatically affect its morbidity. Anesthesiologists have a unique opportunity to be involved in the care of traumatic brain injury patients from their admission, through the operative period, and into postoperative intensive care. In this article, we will review current treatment, standards for monitoring, and goals for resuscitation, both intraoperatively and postoperatively. Co-morbidities that affect resuscitation will also be discussed.
Similar content being viewed by others
Introduction
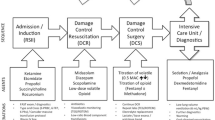
In the United States, incidence of hospital visits and hospitalizations attributed to traumatic brain injury (TBI) have continued to increase over the past decade; however, mortality has decreased. Overall, more than 1.7 million people are affected annually [1]. Traumatic brain injury is normally the result of acceleration or deceleration of the brain by assault, motor vehicle accidents, or falls. Consequences of TBI are classified as cognitive, functional, neuro-electrical, behavioral, and social. The treatment of TBI focuses on reducing morbidity and reducing the neurological consequences for patients. This article reviews the anesthesiologist’s role in caring for the traumatic brain injury patient.
Injury Types
The pathophysiology of traumatic brain injury is dynamic and classified into two distinct periods, primary and secondary. Primary injury is due to force transmitted to the brain immediately following trauma. A list of primary injury types can be found in Table 1 [2].
Secondary brain injuries are caused by systemic derangements, increase morbidity of the primary injury, and are potentially preventable. The major determinants contributing to secondary brain injury include hypotension, hypoxemia, and hypercarbia that result in changes to the intracerebral environment. Table 2 lists sequelae of secondary brain injury [3, 4, 5•].
Initial Treatment
Diagnosis
Initial Glasgow Coma Score (GCS) is commonly used to classify TBI, as it is easy to use and has interobserver reliability [6]. A score of 13–15 is considered mild, 9–12 is considered moderate, and less than 9 is considered severe. Rapid GCS assessment is often obtained before intubation so that immobility secondary to the administration of neuromuscular blocking agents does not become a concern. Computed tomography (CT) scan is most often utilized to formally diagnose traumatic brain injury.
Invasive Monitors
After diagnosis of TBI, consideration should turn toward monitoring. The Monro-Kellie doctrine summarizes that the cranial compartment is incompressible with a fixed volume. Therefore, any increase in volume of one of the cranial constituents must be compensated by a decrease in volume of another to maintain equilibrium. For example, brain swelling will result in reduced blood flow, potentially worsening ischemia and resulting in infarct. Morbidity with TBI is linked to elevated intracranial pressure (ICP); therefore, monitoring ICP becomes paramount. When GCS is 8 or less or the patient cannot follow serial neurological exams, an ICP monitor is often utilized.
Current guidelines recommend an ICP less than 20 mmHg and cerebral perfusion pressure (CPP) greater than 60 mmHg; however, patient-specific variability exists and has been identified [7]. As a result, a targeted approach using patient-specific cerebral autoregulation has been suggested. Averaged values of ICP and mean arterial pressure (MAP) result in a pressure reactivity index (PRx). Plotting PRx against CPP produces a U-shaped curve, with the two ends demonstrating hypoperfusion or low CPP and hyperperfusion or high CPP; both associated with worse cerebrovascular reactivity. The lowest part of the curve is the bottom of the U and is designated the optimal CPP. Factors that may render this measurement unusable include the use of vasoactive medication, high-dose sedation, neuromuscular blockade usage, and decompressive craniectomy that makes the cerebral pressure—volume curve artificially flat [8••]. When direct ICP measurements are not yet available, a mean arterial pressure of 80 mmHg or greater is recommended based on the assumption of an ICP or 20 mmHg.
As an ICP monitor, ventriculostomies are both diagnostic and therapeutic. A ventriculostomy provides the ability to both drain cerebrospinal fluid and measure ICP. Placing a ventriculostomy involves cannulating the ventricle via a durotomy and is the most commonly used ICP monitor in trauma. Ventriculostomies have the highest risk of intracerebral hemorrhage. Another ICP monitor, the Richmond bolt, is seated adjacent to the dura. This device does not penetrate brain tissue, and is diagnostic only. Richmond bolts have been associated with infection, epidural bleeding, and focal seizures. Additionally, epidural intracranial pressure monitors use pneumatic switches that deform as the dura changes; however, these can be difficult to place, calibrate, and can cause bleeding. Finally, the Integra Camino monitor (Plainsboro, NJ, USA), an intraparenchymal intracranial pressure monitor, is a cannula that is inserted directly into the cortical gray matter and directly measures tissue pressure. Camino monitors have a low risk of infection or leak and occlusion of the catheter is rare; however, after placement there is no way to recalibrate the cannula [9]. In addition to measuring ICP, cerebral perfusion pressure (CPP), cerebral blood flow from cortical blood flow, and brain tissue PO2 can be measured with a variety of instruments. The multimodal approach versus the single approach of ICP measurement may result in superior outcome [10].
Ventilation
Early management of traumatic brain injury hinges on the avoidance of secondary insult. Hypoxia is well established to worsen the outcome of TBI. As a result, rapid sequence intubation (RSI) in the field was common practice. Recent studies, however, demonstrated increased mortality with paramedic intubation of brain-injured patients, possibly due to increased transport times, transient hypoxia during the procedure, and inadvertent hyperventilation [11]. Accordingly, patients are transported directly to a trauma center when feasible, aka “scoop and run”. If there is airway compromise in route, patients will divert to a local hospital to obtain a definitive airway and then continue to a suitable trauma center. Patients with severe TBI should be directed to a Level I or II trauma center even if that hospital is not the closest [12].
If not intubated in the field, patients are often intubated after arrival to the trauma center once initial GCS is established. They remain intubated and mechanically ventilated for the intraoperative and postoperative period. After intubation, hyperventilation should be avoided because it causes vasoconstriction of the cerebral circulation and should only be used in short duration for impending herniation. A PaCO2 of less than 25 mmHg is not recommended. A decrease in PaCO2 can reduce ICP by cerebral vasoconstriction; however, it also increases the volume of severely hypoperfused tissue in the injured ischemic brain tissue and results in more ischemia. Protective low tidal volumes and moderate positive end-expiratory pressure (PEEP) can prevent acute lung injury (ALI) that can accompany TBI. In euvolemic patients with previously healthy lungs, PEEP up to 15 cm H2O does not significantly affect intracranial pressures, but the lowest PEEP to maintain open alveoli and avoid hypoxemia should be utilized [4].
Hemodynamics
Hemodynamic goals for the patient with TBI should be weighed against the effects on other injuries. Cerebral perfusion pressure can be used to guide resuscitation. A CPP below 50 mmHg can lead to cerebral infarction in the injured brain and above 70 mmHg is also not recommended [13]. Traumatic brain injury rarely occurs in isolation, requiring the anesthesiologist to have a “whole patient” approach to resuscitation. Increasing the arterial blood pressure to gain an increase in CPP may have a deleterious effect on the patient’s other injuries and a multimodal approach to monitoring is recommended.
Temperature
Therapeutic hypothermia is controversial. If the patient has not suffered a cardiac arrest, patients are rarely cooled. In select populations, studies have shown that hypothermia applied over 48 h can improve outcome; however, the success may be dependent on timing of onset, temperature at admission, rewarming rate which can cause rebound intracranial hypertension, and target temperature. Therefore, the use of hypothermia remains optional [14–16].
Medical Management
Increased ICP can be managed medically and surgically. In a 2010 survey of 295 practitioners by the Neurocritical Care Society, about 90 % reported the use of osmotically active agents to decrease ICP [17].
Mannitol
Mannitol, an osmotically active derivative of mannose, is frequently used in the treatment of elevated ICP since its introduction in the 1960s. It is a level II recommendation at doses of 0.25 gm/kg to 1 g/kg body weight. Its osmotic load establishes a gradient that results in the movement of water from the brain and into the circulation, effectively decreasing brain volume and ultimately leading to a decrease in ICP. Additionally, it improves rheology within minutes of its administration by volume expansion, decreasing blood viscosity and increasing the deformability of red blood cells, and ultimately improving CBF [16, 18, 19]. Despite these positive influences on ICP, mannitol can result in a rebound increase in ICP when the therapy is discontinued since it can penetrate the injured BBB and reside within the injured brain. Mannitol can also lead to complications such as hemolysis, hypotension from its action as an osmotic diuretic, renal failure, CHF and pulmonary edema, and electrolyte imbalances with hyponatremia first from volume expansion and then hypernatremia from hypovolemia [19].
Hypertonic Saline
The use of hypertonic saline (HTS) has steadily increased since its introduction in the early 1990s. A 1995 survey showed 83 % of centers in the US used mannitol to treat increased ICP; however, by 2010 there was a narrowing margin of preference with 55 % preferring HTS and 45 % preferring mannitol [17, 20]. Reasons for increased preference of HTS include less rebound edema, longer lasting effect, and fewer systemic side effects.
HTS is formulated in concentrations of 2, 3, 7.5, 23.4 %. HTS is one of the first line therapies included in the armamentarium at the R Adams Cowley Shock Trauma Center in Baltimore. It is administered readily to those patients who have suffered polytrauma including head trauma with a high concern for elevated ICP. A patient who arrives with head trauma and signs and symptoms indicative of elevated ICPs will receive 500 ml of 3 % HTS in addition to other appropriate interventions. HTS, by restoring circulating blood volume, improves cardiac output, perfusion and MAP in hypovolemic trauma patients, improving CBF while minimizing the risk of increasing cerebral edema through the administration of large volumes of NS or other crystalloids.
With the goal of preserving CPP by decreasing ICP and elevating MAP, further secondary injury due to ischemia may be minimized. Therefore, HTS has been compared to mannitol in numerous studies. Elevations of ICP greater than 20 mmHg treated with equimolar doses of 20 % mannitol and 15 % HTS administered as a bolus have shown a mean decrease in ICP of 7.96 versus 8.43 mmHg, respectively, with HTS producing a longer duration of the reduction [21]. In patients with severe ICP elevation, generally greater than 30 mmHg, HTS has been shown to generate more significant reductions in ICP [22]. Cumulative ICP burden is defined as the total number of days in which patients had an elevation of ICP greater than or equal to 25 mmHg from the total number of days in which ICP was monitored. A recent retrospective analysis showed that patients who received HTS had a significantly lower cumulative ICP burden and a statistically significant decrease in the daily ICP burden than those receiving mannitol [23•].
When HTS is used, serum sodium and serum osmolality should be followed. Generally, serum Na+ should be maintained less than 162 mOsm/L and serum osmolality under 382 mOsm/L [22]. Complications associated with HTS are renal failure, coagulopathy, pulmonary edema, hypernatremia, hyperkalemia, central pontine myelinolysis, hyperchloremic metabolic acidosis, hyperoncotic hemolysis, vein sclerosis if high concentrations are administered through a peripheral vein, and rebound intracranial hypertension although this is less than the risk with mannitol [17, 22].
Albumin
Whether a patient suffering from both traumatic brain injury and hypovolemic shock should receive albumin has not yet been completely elicited. According to the SAFE trial, TBI patients receiving 4 % albumin had a significantly higher risk of death compared to those receiving saline in the ICU. Patients with severe TBI receiving 4 % albumin have a 1.6 relative higher risk of death at 24 months compared to patients receiving saline, despite the lack of differences in hemodynamic resuscitation end points; however, the albumin group did have higher initial intracranial pressure. Possible explanation is that the use of albumin could lead to worsening of cytotoxic or vasogenic cerebral edema [24]. 4 % Albumin has a calculated osmolarity of 274.5 mOsml/L and a measured osmolality of 266 mOsm/kgH2O, and isotonic saline has an osmolarity of 308 mOsm/L with a measured osmolality of 285 mOsm/kg H2O. Therefore, the relative hypotonicity of 4 % albumin could contribute vasogenic edema [25]. The sulfhydryl group on albumin can interact with nitrous oxide and limit its breakdown, ultimately decreasing cerebral vasodilation [26]. With conflicting data, there is no definitive guideline for the use of albumin in polytrauma TBI patients.
Pentobarbital
In addition to the therapies discussed above, pentobarbital coma can also be induced in patients suffering from intractable intracranial HTN.
Seizure Prophylaxis
The traumatized brain is also at high risk for developing seizures. In those who suffer mild TBI, the relative risk of developing seizures is double to those without TBI. In those who suffer intracranial hypertension or brain contusion, the risks rise by 5- to 6-fold. For those who suffer a combination of intracranial hypertension and contusion, a 43-fold increase in post-traumatic seizures can be seen. [27•]. Levetiracetam is commonly used for seizure suppression along with phenytoin. Anti-seizure medications are maintained for at least 7 days after severe traumatic brain injury [28].
Surgical Management
Decompressive Craniectomy
Management of TBI hinges on controlling ICP. Patients who require surgical intervention due to refractory intracranial hypertension despite medical treatment often undergo emergency decompressive craniectomy that involves releasing the pressure by removing a part of the skull. Patients with a higher GCS score on admission have better outcomes after decompressive craniectomy, have a shorter ICU stay, and require the ventilator for shorter periods [29, 30]. Early decompressive craniectomy, utilized commonly at R Adams Cowley Shock Trauma, has been shown to have good functional outcomes in patients with severe TBI [31, 32]. Some indications used to indicate the need for a decompressive craniectomy include unilateral or bilateral cerebral edema, midline shift greater than 5 mm with a GCS of 8 or less, worsening neurological exam, bilateral fixed pupils with intact brainstem reflex, intractable intracranial HTN with ICP greater than 25 mmHg, and cerebral edema despite hematoma evacuation [33].
Intraoperative management of patients undergoing emergency decompression includes obtaining large bore intravenous access for blood and possible vasopressor administration. Commonly 16- or 14-gauge intravenous catheters are placed. Rapid infuser catheters or central access can also be established. An arterial catheter is placed for constant blood pressure monitoring. Patients usually arrive to the operating room with intracranial monitors already established, since this is one indicator for the need of the operation. As a result, an arterial line and ICP monitor allow CPP to be calculated and followed. Blood products should be readily available, including fresh frozen plasma, cryoprecipitate, and possibly prothrombin complex concentrate (PCC) to reverse chemical anticoagulation and trauma-induced coagulopathy. There is some uncertainty on the utility of tranexamic acid (TXA) in patients with traumatic brain injury. It has been suggested that TXA decreases the progression of intracranial hemorrhage and reduces, although not significantly, the clinical outcome of TBI patients [34••]. There was no increase in mortality with its use and can be administered when bleeding cannot be controlled or a thromboelastogram demonstrates fibrinolysis [35•].
Decompressive Laparotomy
Despite all other medical and surgical interventions, some patients continue to have an elevated ICP. For those patients who have failed aggressive medical and surgical intervention, including a decompressive craniectomy, a study at the R Adams Cowley Shock Trauma Center showed a decompressive laparotomy which resulted in a 65 % survival rate [36]. Intracranial, intrathoracic, and intra-abdominal compartments are not isolated and inherently affect one another by transmission of pressures through the venous system. Elevations in the intra-abdominal compartment especially after massive resuscitation in multiply injured trauma patients can result in elevation of ICP and release of the abdominal pressure can lead to drastic and significant reductions in ICP [36, 37].
Co-morbid Conditions Associated with TBI
Aside from surgical complications, TBI carries the risk of many complications. In addition to post-traumatic seizures, the risk of developing hydrocephalus, spasticity, gait abnormalities, agitation, and gastrointestinal and genitourinary disorders are high. A myriad of immediate systemic complications including cardiovascular, pulmonary, hematologic, immunologic, and endocrinologic dysfunction can occur [38].
Cardiopulmonary
Abnormalities in cardiac conduction include tachycardia, ST segment changes with and without elevated cardiac troponins, QTc prolongation, abnormal T waves, and U waves. Wall motion abnormalities can also be seen, including Takotsubo cardiomyopathy or broken heart syndrome characterized by paradoxical septal and posterior wall motion between mid-left ventricle and apex on echocardiogram [38]. Recognition of the high risk of developing these cardiovascular abnormalities is crucial in the management of TBI patients. Pulmonary complications including aspiration pneumonitis, lower respiratory tract infections, acute respiratory distress syndrome (ARDS), ventilator-induced lung injury (VILI), and neurogenic pulmonary edema can develop and make intraoperative and postoperative management of respiratory status difficult [38]. Using ICU ventilators, with a greater variety of ventilator modes, for transport and intraoperatively, is a common strategy to reducing or managing the pulmonary complications. Recognizing these complications and being prepared to address them are key in optimizing oxygenation and ventilation.
Hematologic and Immunologic
Pathophysiologic mechanisms for hematologic abnormalities associated with TBI are not well understood and can present as fibrinolysis, disseminated intravascular coagulation (DIC), platelet dysfunction, thrombocytopenia, and hypercoagulability. These derangements can result in an increased incidence of deep vein thrombosis, microthrombosis, and ischemia. One mechanism for the development of coagulopathy is associated with the massive release of tissue factor from the injured brain leading to overactivation of the extrinsic pathway for coagulation, ultimately resulting in a consumptive coagulopathy [38–40]. The risk of having such abnormalities is very high within the first 24 h of injury, but it can persist for 3 or more days thereafter [39]. Recognition of this is important in the intraoperative management and postoperative management of these patients since many TBI patients return for multiple surgical procedures during their admission.
TBI patients are at higher risk of acquiring nosocomial infections due to an increased activity of immunosuppressive cytokines (IL 4, IL 10, transforming growth factor β) compared to pro-inflammatory activity [41]. Maintaining any perioperative antibiotics chosen by the ICU or infectious disease specialists must be prioritized in such patients.
Neuroendocrinologic
SIADH is a well-known complication of TBI and presents as euvolemic hyponatremia with hypoosmolar serum and hyperosmolar urine. It is treated with fluid restriction, hypertonic saline, and fludrocortisone to a goal of sodium correction 0.5–1 mmol/l per hour. Post-traumatic diabetes insipidus, seen in approximately 3–51 % of patients, is seen within the first few days following brain injury and is commonly seen as hypotonic, voluminous urine of >200 ml/h for 2 consecutive hours or >5 ml/kg/h in a hypovolemic, hypernatremic patient [41]. It is usually transient, lasting for a few days to a few weeks. Intraoperative and postoperative recognition of these issues is important and should be communicated with the intensivist.
Conclusions
Traumatic brain injury is a complex and evolving entity. It requires knowledge of underlying processes such as mechanism of injury and the requirements to prevent or limit a potentially devastating secondary injury. Multiple modalities exist to facilitate return to function after traumatic brain injury including initial treatment such as diagnosis, blood pressure control, and intracranial pressure monitoring and control; medical management with mannitol and hypertonic saline; surgical control through decompressive craniotomy or laparotomy; and postoperative management including seizure prophylaxis. Patient-specific techniques, new monitors, labs, and drugs continue to evolve to decrease the morbidity and mortality of traumatic brain injury.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Injury Prevention and Control: Traumatic brain injury. Cdc.org 2014.
Wilson WC. Traumatic brain injury. Trauma, vol. 2. New York: Informa Healthcare; 2007.
Graham DI, Adams JH, Nicoll JA, et al. The nature, distribution and causes of traumatic brain injury. Brain Pathol. 1995;5:397–406.
Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12.
• Rosenfeld JV, Maas AI, Bragge P, et al. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–98. An expansive review of the causes of secondary brain injury.
Kraus JF, McArthur DC. Chapter 1. Neurology and trauma. 2nd ed. Oxford: Oxford University Press; 2006.
Sl Bratton, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. Neurotrauma. 2007;24(Suppl 1):S59–64.
•• Weersink CSA, Aries MJH, Dias C, et al. Clinical and physiological events that contribute to the success rate of finding “optimal” cerebral perfusion pressure in severe brain trauma patients. Crit Care Med. 2015;43:1952–63. Hypoperfusion and hyperperfusion were both found to be detrimental to cerebrovascular reactivity. This paper describes a new concept in targeting cerebral autoregulation to avoid both and maintain an optimal cerebral perfusion pressure.
Wilson WC. Neurological monitoring. Trauma, vol. 2. New York: Informa Healthcare; 2007.
Isa R, Wan Adnan WA, Chazali G, et al. Outcome of severe traumatic brain injury: comparison of three monitoring approaches. Neurosurg Focus. 2003;15:E1.
Davis DP, Hoyt DB, Ochs M. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma. 2003;54:444–53.
Hartl R, Gerber L, Iacono L, et al. Direct transport within an organized state trauma system reduces mortality in patients with severe traumatic brain injury. J Trauma. 2006;60:1250–6.
Prabhakar H, Sandhu K, Bhagat H, et al. Current concepts of optimal cerebral perfusion pressure in traumatic brain injury. J Anaesthesiol Clin Pharmacol. 2014;30:318–27.
Davies AR. Hypothermia improves outcome from traumatic brain injury. Crit Care Resusc. 2005;7:238–43.
Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71.
Guidelines for the management of severe traumatic brain injury. 3rd ed. J Neurotrauma 2007;24:S1.
Hays AN, Lazaridis C, Neyens R, et al. Osmotherapy: use among neurointensivists. Neurocrit Care. 2011;14:222–8.
Diringer MN. New trends in hyperosmolar therapy? Curr Opin Crit Care. 2013;19:77–82.
Grande PO, Romner B. Osmotherapy in brain edema: a questionable therapy. J Neurosurg Anesthesiol. 2012;24:407–12.
Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury (Review). Cochrane Collab. 2013;8:1–24.
Sakellaridis N, Pavlou E, Karatzas S, et al. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J Neurosurg. 2011;114:545–8.
Kerwin AJ, Schinco MA, Tepas JJ, et al. The use of 23.4 % hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67:277–82.
• Mangat HS, Chiu YL, Gerber LM, et al. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg. 2015;122:202–10. Hypertonic saline and mannitol are both utilized to decrease intracranial pressure in TBI patients, however it is unclear which is superior. This small study compared the two to determine the most effective.
Myburgh J, Cooper DJ, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–84.
Van Aken HK, Kampmeier TG, Ertmer C, Westphal M. Fluid resuscitation in patients with traumatic brain injury: what is a SAFE approach? Curr Opin Anesthesiol. 2012;25:563–5.
Baker AJ, Park E, Hare GM, et al. Effects of resuscitation fluid on neurologic physiology after cerebral trauma and hemorrhage. J Trauma. 2008;64:348–57.
• Mahler B, Carlsson S, Andersson T, et al. Unprovoked seizures after traumatic brain injury: a population-based case-control study. Epilepsia. 2015;56:1443–4. Patients with TBI are at higher risk of seizure. This large population study quantified the risk of seizure and also the timing at which this risk was greatest.
Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502.
Aarabi B, Simard JM. Traumatic brain injury. Curr Opin Crit Care. 2009;15:548–53.
Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;16:1493–502.
Howard JL, Cipolle MD, Anderson M, et al. Outcome after decompressive craniectomy for the treatment of severe traumatic brain injury. J Trauma. 2008;65:380–6.
Williams RF, Magnotti LJ, Croce MA, et al. Impact of decompressive craniectomy on functional outcome after severe traumatic brain injury. J Trauma. 2009;66:1570–6.
Seung PB, Young-Je S, Hee-Jin Y, et al. Analysis of complications following decompressive craniectomy for traumatic brain injury. J Korean Neurosurg Soc. 2010;48:244–50.
•• Zehtabchi S, Baki SAG, Falzon L, Nishijima DK. Tranexamic acid for traumatic brain injury: a systematic review and meta-analysis. Am J Emerg Med. 2014;32:1503–9. Tranexamic acid has been studied for reducing the progression of intracranial hemorrhage. This meta-analysis of 1030 results found two randomized controlled trials that met criteria and showed statistically significant reduction in hemorrhage, giving providers another method to treat hemorrhage due to traumatic brain injury.
• Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, et al. Tranexamic acid for patients with traumatic brain injury: a randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013;13:20. This randomized, double-blinded, placebo-controlled study, although small, showed that tranexamic acid was safe to use, with no increased risk of thromboembolic events and trended toward a reduction in intracranial hemorrhage progression. It provided a start for future larger studies.
Scalea TM, Bochicchio GV, Habashi N, et al. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: multiple compartment syndrome. J Trauma. 2007;62:647–56.
Joseph DK, Dutton RP, Aarabi B, Scalea TM. Decompressive laparotomy to treat intractable intracranial hypertension after traumatic brain injury. J Trauma. 2004;57:687–95.
Wijayatilake DS, Sherren PB, Jigajinni SV. Systemic complications of traumatic brain injury. Curr Opin Anesthesiol. 2015;28:525–31.
Laroche M, Kutcher ME, Huang MC, et al. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70:1334–45.
Meagle M. Coagulopathy after traumatic brain injury: incidence, pathogenesis and treatment options. Transfusion. 2013;53:28S–37S.
Capatina C, Paluzzi A, Mitchell R, Karavitaki N. Diabetes insipidus after traumatic brain injury. J Clin Med. 2015;4(7):1448–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Anesthesia for Trauma.
Rights and permissions
About this article
Cite this article
Conti, B., Villacin, M.K. & Simmons, J.W. Trauma Anesthesia for Traumatic Brain Injury. Curr Anesthesiol Rep 6, 95–101 (2016). https://doi.org/10.1007/s40140-016-0141-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-016-0141-1