Abstract
Female pelvic floor dysfunction encompasses a number of prevalent clinical conditions, including female pelvic organ prolapse, urinary and fecal incontinence, obstructed defecation, and sexual dysfunction. In most cases, neither etiology nor pathophysiology are well understood. Imaging has great potential to enhance both our research and clinical management capabilities in this field, and to date this potential is underutilised. Of the available techniques such as X-ray, computed tomography, magnetic resonance imaging, and ultrasound, the last is generally superior for pelvic floor imaging, especially in the form of perineal or translabial imaging. The technique is safe, simple, cheap, and easily accessible, and provides high spatial and temporal resolutions. Translabial or perineal ultrasound is useful in determining residual urinary volume, detrusor wall thickness, and bladder neck mobility, and in assessing pelvic organ prolapse as well as levator function and anatomy. It is also useful for the evaluation of obstructed defecation and fecal incontinence. It is at least equivalent to other imaging techniques in diagnosing conditions as diverse as urethral diverticulum, rectal intussusception, and avulsion of the puborectalis muscle. Ultrasound is currently the only imaging method capable of visualizing modern slings and mesh implants, and it may help in selecting patients for prolapse mesh surgery by identifying those most at risk of recurrence. Birth trauma to the levator ani muscle seems to be the central etiological factor for pelvic organ prolapse and recurrence after prolapse surgery, and it is easily diagnosed by pelvic floor ultrasound. Similarly, delivery-related trauma to the external and internal anal sphincter is central to the aetiology of anal incontinence in women and is equally easily diagnosed using either endo- or exoanal (translabial or perineal) ultrasound. This review will focus on translabial/perineal ultrasound as the least invasive, cheapest, simplest, and most commonly available method for pelvic floor imaging. I will discuss the main current uses of the method in clinical practice and research, and will try and provide a perspective for likely future developments in this field.
Similar content being viewed by others
Introduction
Ultrasound has been commonly used in clinical practice for well over one generation, and most gynecologists and urologists have been exposed to its application during their training. Increasingly, this is also the case for colorectal surgeons, although most surgical colleagues use endoanal rather than translabial ultrasound, and most would ask for a defecation proctography or MR defecography rather than translabial ultrasound when the investigation of obstructed defecation is required. In gynecology, it took about 30 years for transvaginal ultrasound to become commonly accepted in the detection and evaluation of pelvic masses, pelvic pain, and infertility. Ultrasound imaging in urogynecology for pelvic organ prolapse (POP), lower urinary tract, and defecatory symptoms is about halfway through this process of increasing acceptance. Radiological techniques have been used since the 1920s to describe bladder appearance and descent [1], and, later, also for central and posterior compartment prolapse [2]. The advent of B mode real-time ultrasound has made sonography an obvious alternative, whether via the transperineal [3, 4] (see Fig. 1) or the vaginal route [5]. Magnetic resonance imaging (MRI) has also become an option since the 1990s, but the lack of dynamic imaging capability, limited availability, and cost issues have slowed its general acceptance.
Transducer placement on the perineum (left), and schematic representation of imaged structures in the resulting midsagittal plane. (Reprinted from Dietz [107]; copyright 2010, with permission from Elsevier.)
The constantly growing interest in pelvic floor imaging is attributable to a number of distinct factors. Female pelvic floor dysfunction encompasses several highly prevalent conditions. The estimated lifetime risk of surgery for POP or urinary incontinence in women is quoted as 10–20 % [6, 7]. Despite the high prevalence, however, our understanding of the aetiology and pathophysiology of female pelvic floor dysfunction is limited, resulting in largely empirical treatment without solid scientific basis. This is reflected in a high re-operation rate of about 1 in 3 [6].
The recent re-discovery of the link between childbirth-related pelvic floor muscle trauma and female POP is a good example of how imaging may help us understand the pathophysiology of pelvic floor dysfunction [8–11, 12•], allowing primary [13•] and secondary prevention trials and hopefully resulting in effective treatment based on translational research [14, 15]. It is more and more obvious that clinical assessment alone is insufficient to assess pelvic floor function and anatomy. Our clinical examination generally focuses on the description of surface anatomy which is often unable to reveal true underlying structural abnormalities. To give one example related to the diagnosis of prolapse of the posterior vaginal compartment, which is common in women with symptoms of prolapse and obstructed defecation: gynecologists call posterior vaginal wall descent a ‘rectocele’, but this appearance may be caused by at least five distinct anatomical conditions which are difficult to distinguish without imaging. These include true radiological rectocele, perineal hypermobility, enterocele, rectoenterocele, and rectal intussusception [16, 17]. Colorectal surgeons commonly avoid this particular pitfall by employing defecation proctography, but this method involves radiation exposure, is very unpleasant for the patient, and is unable to demonstrate problems in other compartments and abnormalities of the levator ani, all of which are common in women with obstructed defecation.
Of more importance in the realm of the gynecologist is the fact that a clinical assessment for female POP is to a large extent determined by confounders that are commonly overlooked, such as bladder [18] and rectal filling, levator co-activation [19], and the duration of a Valsalva maneuver [20]. It is not surprising that findings in the operating theatre often vary from those obtained in the outpatient setting, since all potential confounders act to increase the likelihood of false-negative results. Hence, one should ensure bladder (and, if possible, rectal) emptying, a sufficiently long maximal Valsalva of at least 5 s, and relaxation of the levator ani. Real-time imaging makes it much easier for the operator to avoid these confounders by using visual biofeedback.
This review is limited to translabial/perineal ultrasound imaging. Some investigators prefer to use ‘introital ultrasound’, a technique involving the use of front-firing vaginal endo-probes placed in the vaginal introitus. Such probes allow higher resolutions, but there are limitations in assessing the effect of maneuvers and in imaging of the levator ani, which is why I am not going to discuss this technique further. However, most of the issues described here also apply to introital ultrasound.
Instrumentation and Basic Methodology
The basic requirements for perineal or translabial imaging include a B-mode capable 2-dimensional (2D) ultrasound system with cine-loop function, a 3.5- to 6-MHz curved array transducer and a video-printer. Such systems can be obtained at low cost and are commonly available, as they are widely used for imaging of the fetus or the paediatric and adult abdomen. The examination is performed in dorsal lithotomy, with hips flexed and slightly abducted and the heels placed close to the buttocks, or in the standing position.
A full bladder, or sometimes a full bowel, can prevent complete development of prolapse, which is why imaging is best performed after voiding, or after catheterisation if there is voiding dysfunction. Fecal impaction may necessitate repeat scanning after bowel emptying. In preparation for scanning, gel is applied to the probe which is then covered with a powder-free glove, condom, a commercial probe cover, or thin plastic wrap. One should avoid air trapping under the probe cover as this impairs image quality. The probe is then placed on the perineum, resulting in a midsagittal view (see Fig 1). Imaging conditions are superior in pregnancy and poorest in menopausal women with marked atrophy, likely due to variations in tissue hydration. Hypertrophic or hirsute labia should be parted. Scar tissue may impair visibility, but obesity is rarely an issue. The probe can be placed quite firmly on perineum and symphysis pubis without causing discomfort, except in women with marked atrophy or vulvitis. On Valsalva, however, one should not exert undue pressure on the perineum to allow full development of pelvic organ descent. The standard mid-sagittal view demonstrates, from ventrally to dorsally, the symphysis pubis, urethra and bladder neck, vagina, uterine cervix, and anorectal junction (see Fig. 1). Dorsal to the anorectal junction, the central portion of the levator plate, i.e. the puborectalis muscle, is identified. Parasagittal planes can yield additional information, e.g., in confirming urethral integrity and enabling assessment of the puborectalis muscle [21]. An oblique coronal view, obtained by rotating the probe by 90 ° and inclining it dorsally towards the anal canal also allows assessment of the anal sphincter. In order to obtain sufficient distance between the sphincter and the transducer, it is best to move the transducer more ventrally and tilt it to a more vertical position until the sphincter is visualized at a depth of 1–2 cm (see Fig. 2). A deficient perineum may make this more difficult.
There is no consensus on image orientation. The first published translabial images showed the perineum at the top and the symphysis pubis on the left [3, 4] or the same turned on its head, i.e., rotated by 180 ° [22]. The author prefers the original orientation as on conventional transvaginal ultrasound, i.e. cranio-ventral aspects to the left, dorsocaudal to the right (see Fig. 1). This orientation is highly convenient when using 3-dimensional (3D)/4-dimensional (4D) systems as shown in Fig. 3. The top left image demonstrates the midsagittal plane, and the bottom right a rendered volume in the axial plane for visualizing the levator ani muscle and hiatus.
Standard acquisition screen of 3D pelvic floor ultrasound when using an abdominal 3D/4D transducer. The midsagittal plane is shown in (a), the coronal plane in (b), the axial plane in (c). A rendered axial plane (i.e., a semitransparent representation of all voxels in the box seen in a–c) is shown in d. S symphysis pubis, U urethra, V vagina, R rectal ampulla, A anal canal, P puborectalis muscle. (Reprinted from Dietz [110]; with permission.)
After completing the examination, the probe is freed of gel by mechanical cleaning, followed by disinfection with alcoholic wipes. Sterilization as for endoprobes is usually considered unnecessary. Table 1 shows a list of indications for pelvic floor ultrasound imaging.
Urinary Incontinence
Ultrasound is commonly used to assess residual urinary volume after voiding. Using the transperineal route the bladder volume in millilitres can be estimated by using the formula X cm × Y cm × 5.6 [23] with X and Y describing maximal bladder dimensions in the midline, measured perpendicular to each other (Fig. 4). The method can also detect foreign bodies or bladder tumors [24, 25]. In the investigation of urinary incontinence. detrusor wall thickness may be of interest, since it is associated with detrusor overactivity [26]. Unfortunately, detrusor hypertrophy is sometimes rather variable as shown in Fig. 4, resulting in limited repeatability, which probably contributes to the fact that the association is not strong enough to replace urodynamic testing [27–29]. Unlike in the male, bladder wall thickness was not found to be predictive of voiding difficulty in women [30, 31].
Pelvic floor ultrasound, midsagittal plane. Measurement of residual urine and detrusor wall thickness. S symphysis pubis, U urethra, V vagina, A anal canal, R rectal ampulla, D detrusor muscle. The detrusor shows highly asymmetrical hypertrophy which is not uncommon in detrusor overactivity. The horizontal and vertical lines illustrate maximal bladder diameters obtained for the estimation of residual urine volume [23]
Translabial ultrasound has long been used in the evaluation of stress urinary incontinence. Due to the assumption that urethral hypermobility is important in the etiology of female stress urinary incontinence, bladder neck mobility was one of the first proposed indications for ultrasound imaging in urogynaecology. On Valsalva, the proximal urethra can be seen to rotate postero-inferiorly around the fulcrum of the symphysis pubis. To measure bladder neck position and mobility, points of reference are either the central axis of the symphysis pubis [32] or its infero-posterior margin [33], with the latter more practicable due to transducer dimensions and calcification of the interpubic disc in older women.
Bladder neck position and mobility can be measured with high reliability [34] by determining bladder neck position relative to the symphysis pubis at rest and on maximal Valsalva (see Fig. 5). The difference yields a figure for bladder neck descent (BND). It is in fact mobility of the mid-urethra, not of the bladder neck, that is most predictive of stress incontinence, and segmental urethral mobility can also be determined with limited effort, especially when using 4D volume ultrasound datasets [35–38]. Urethral rotation can be measured in the same plane by comparing the angle of inclination between the proximal urethra and the symphyseal axis. Some investigators also assess the urethrovesical angle between the proximal urethra and trigone. However, translabial ultrasound imaging is not sufficient to replace urodynamic testing for the diagnosis of urinary stress incontinence [39, 40].
Determination of bladder neck descent and retrovesical angle: Ultrasound images show the midsagittal plane at rest (a, c) and on Valsalva (b, d). S symphysis pubis, U urethra, B bladder, Ut uterus, V vagina, A anal canal, R rectal ampulla, L levator ani. The lower images demonstrate the measurement of distances between inferior symphyseal margin and bladder neck (vertical, x; horizontal, y). and the retrovesical angle at rest (rva-r) and on Valsalva (rva-s). (Reprinted from Dietz [49••]; copyright 2011, with permission from Springer.)
Female Pelvic Organ Prolapse
Female pelvic organ prolapse (FPOP) is a common indication for surgical treatment, with a lifetime risk of between 10 and 20 % [6, 7]. Most prolapse procedures are performed by gynecologists and urogynecologists, but urologists and colorectal surgeons also undertake such surgery. The aetiology of FPOP is likely to be multifactorial and is not completely understood as yet [41]. Congenital factors clearly play a role [42], and lifestyle factors such as obesity and asthma are said to contribute. However, pregnancy and childbirth, especially vaginal delivery, are the commonest and most substantial modifiable risk factors. The relative contribution of factors appears to vary for different forms of prolapse: childbirth-related pelvic floor trauma seems to play only a minor role in the aetiology of rectocele [43, 44], but it is of paramount importance for uterine and bladder prolapse [45, 46], likely at least partly mediated through the increasingly well-defined factor of levator trauma [9, 11] (see below).
The severity or extent of FPOP is conveniently quantified against the postero-inferior margin of the symphysis pubis (Fig. 6). Ultrasound imaging for prolapse quantification has proven particularly useful in outcome assessment after pelvic reconstructive surgery, both clinically and in research, and it has led to a re-appraisal of what we mean by ‘prolapse’. Gynecologists often use the word ‘cystocele’ when we really mean ‘anterior vaginal wall descent’, and ‘rectocele’ when we ought to say ‘posterior vaginal wall descent’. Anterior vaginal wall descent can be due to several pathologic entities such as cystocele, urethral diverticulum, Gartner duct cyst, or even an anterior enterocele [47••]. Ultrasound can distinguish these conditions, which is difficult to impossible on clinical examination. Urethral diverticula are commonly overlooked in women with recurrent urinary tract infections, frequency, urgency, and dysuria [48]. Urethral structure and spatial relationships are particularly well delineated in a multi-axial view such as in Fig. 7, and the axial plane is most useful in distinguishing a urethral diverticulum from the main differential diagnosis, a Gartner duct cyst [49••].
Prolapse quantification on translabial ultrasound. The horizontal line of reference is placed through the inferior margin of the symphysis pubis (S) in a still obtained on maximal Valsalva. Vertical lines indicate maximal descent of bladder (B), uterine cervix (Cx), pouch of Douglas (POD) and rectal ampulla (R) relative to the symphysis pubis. There is a cystocele and uterine prolapse; descent of the pouch of Douglas, rectal ampulla and anal canal (A) are all within the normal range
As regards the central compartment, the uterus can at times be difficult to identify because of its iso-echoic nature, similar in echotexture to the vaginal wall, especially in postmenopausal women with atrophic uteri (see Fig. 6). Occasionally ultrasound can show the effect of an anteriorised cervix in women with an enlarged, retroverted uterus, explaining symptoms of voiding difficulty. On the other hand, a mobile acutely anteverted uterus can result in compression or ‘plugging’ of the anorectum, explaining symptoms of obstructed defecation—what radiologists call a ‘colpocele’ on defecation proctography.
Pelvic floor imaging is particularly useful in prolapse of the posterior vaginal wall and obstructed defecation, two conditions that are commonly associated. In this field, collaboration between colorectal surgeons, gastroenterologists, and gynecologists seems to be particularly promising, and there certainly is much that all three specialties can learn from each other in the diagnosis and management of such patients [50••]. Sonographic imaging allows us to distinguish the different anatomical conditions leading to descent of the posterior vaginal wall. The commonest in the author’s own practice is a ‘true rectocele’ where a defect of the rectovaginal septum (RVS) results in herniation of the anterior wall of the rectal ampulla into the vagina. While the RVS is difficult to visualize directly unless one uses endovaginal ultrasound [51], a defect of the septum is implicit in the observation of a discontinuity of the anterior anorectal muscularis on Valsalva maneuver [16]. Another cause of posterior vaginal wall descent is perineal hypermobility where the RVS is intact but abnormally distensible, often combined with an excessively mobile perineum and ‘ballooning’ (see below). Both rectocele and perineal hypermobility are associated with symptoms of prolapse, i.e. the sensation of a vaginal lump or a dragging sensation. On the other hand, symptoms of obstructed defecation such as straining at stool, incomplete bowel emptying, and vaginal digitation only seem associated with a true radiological rectocele, that is, a defect of the RVS [52]. Other causes of posterior compartment prolapse include a combined rectoenterocele, an isolated enterocele, rectal intussusception, or a deficient perineum giving the impression of a ‘bulge’. Rectal intussusception is of particular interest to colorectal surgeons and gastroenterologists and is generally regarded as an early stage of rectal prolapse. In this condition, which is sometimes identified in asymptomatic women, the rectal wall is inverted and enters the proximal anal canal, forcing it open and producing an arrow-shaped distension on Valsalva that is pathognomonic of the condition. Its etiology remains unclear to date, but, rather intriguingly, there seems to be a strong association between abnormal levator hiatal distensibility and levator trauma on the one hand and intussusception on the other [53]. Figure 8 shows a comparison of rectocele, perineal hypermobility, and rectal intussusception in patients who were all clinically diagnosed with a Stage II ‘rectocele’. Figure 9 demonstrates a simple ‘true rectocele’ in the three orthogonal planes and a rendered volume. Images in the coronal and axial plane (b and d) show that this rectocele, as most others, is symmetrical, suggesting a high transverse defect of the rectovaginal septum.
The distinction between a ‘true rectocele’, i.e., a defect of the rectovaginal septum (left), perineal hypermobility (middle), and rectal intussusception (right). All three conditions can manifest as a clinical ‘rectocele’ and are virtually impossible to distinguish on examination. (Reprinted from Dietz [108], with permission from Springer.)
While many women with such anatomical abnormalities of the posterior compartment are asymptomatic, defecatory symptoms are common in patients with pelvic floor dysfunction. Defecation proctography is the gold standard in the investigation of patients with obstructed defecation by imaging specialists, but the technique involves the use of ionising radiation, is invasive, and costly. It is therefore not surprising that colorectal surgeons and gastroenterologists have started using ultrasound [54–56], which is much better tolerated [17] and cheaper, in the initial investigation of women with defecatory symptoms. If there is a rectocele or a rectal intussusception on ultrasound, this condition is very likely to be found on defecation proctography [17, 57•]. It is highly probable that ultrasound will replace radiological techniques in the initial investigation of women with defecatory symptoms [50••, 56].
Of course, diagnostic information only contributes to improved outcomes if it is interpreted correctly and utilized by the clinician. Unfortunately, it is not always clear what kind of therapeutic consequences one should draw from imaging findings such as that of a rectal intussusception. However, one would certainly not expect a rectocele repair to alleviate symptoms due to intussusception. If there is a true rectocele then clearly a defect specific rectocele repair [58] is the surgical treatment of choice. On the other hand, if posterior compartment descent is due to a hyperdistensible fascia as in the case of perineal hypermobility then one is unlikely to find an RVS defect in theatre. Fascial plication or even a levatorplasty may be a better surgical treatment option in such patients. However, this approach would not be expected to resolve symptoms of obstructed defecation, which may be functional in origin. Finally, it seems to make little sense to remove portions of rectal wall, as in the stapled transanal rectal resection (STARR) procedure, and expect cure of symptoms of obstructed defecation in someone who has a herniation of the rectal wall due to a defect of the RVS. Clearly, there is much we still have to learn in the management of patients with posterior compartment prolapse and obstructed defecation.
Fecal Incontinence
Endoanal ultrasound is routinely used to evaluate the anal sphincters, especially in women with fecal incontinence. This involves placement of an ultrasound probe inside the anal canal which distorts anatomy and precludes assessment on sphincter contraction which can enhance tissue definition. Exoanal ultrasound imaging on the other hand, first described by an obstetrician in 1997 [59], does not have these disadvantages. It is now widely used to image the anal sphincter using either endovaginal or transabdominal probes and is especially useful in its 3D/4D incarnation [60–63]. The method has been shown to correlate well with 2D endoanal imaging [64]. It will however be necessary to validate both methods against symptoms in a comparative manner to determine their relative value.
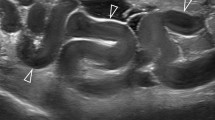
To image the anal sphincter, an electronic curved array transducer is rotated by 90 ° and placed transversely on the perineum (Fig. 2). The probe is then inclined dorsally towards the anal canal to obtain a tilted coronal or near-axial view. The probe should be tilted quite acutely downwards, that is, from ventrocaudally to dorsocranially, in order to gain distance from the sphincter and allow it to be imaged at an optimal depth for focusing in the near field. Imaging is performed on sphincter contraction which enhances the definition of muscular defects. Figure 10 shows tomographic ultrasound imaging (TUI) of a normal anal sphincter (left) and a sphincter with a significant residual defect after primary repair of an obstetric anal sphincter tear (right). Using this technique, a significant defect of the external anal sphincter has been observed in 40 % of patients after primary repair of obstetric anal sphincter injury [65] and in more than 1/4 of primiparous women after vaginal delivery [60]. A significant correlation was found between defecatory symptoms and residual defect of the external anal sphincter as well as levator avulsion [65]. Recent improvements in resolution and tissue discrimination now also allow assessment of the internal anal sphincter. Figure 11 demonstrates an abnormal internal sphincter after haemorrhoidectomy. It is likely that this method will also prove useful in the investigation of other anorectal conditions such as perianal fistula.
Tomographic or multislice imaging facilitates assessment of the anal canal by exoanal translabial ultrasound. A set of eight slices is obtained from the level of the m. puborectalis (Slice 1) to the level of the anus (Slice 8) to bracket the full extent of the external sphincter. A residual or ‘significant’ defect is diagnosed if 4 out of the 6 slices from (2) to (7) show a defect of ≥30 °. The set of images on the left illustrates normal external and internal sphincters; the set on the right shows defects of both external and internal sphincters (asterisk)
Urogynecological Implants
Synthetic implants have become very popular for the surgical treatment of stress urinary incontinence, and recently also for POP, and they have engendered great controversy. Ultrasound is the modality of choice for such implants as they are easily imaged with ultrasound, unlike with MR and X-ray. Since the introduction of synthetic suburethral slings in the 1990s, these have become the gold standard in the surgical treatment of female urodynamic stress incontinence. Ultrasound can confirm the presence of such slings (see Fig. 12) and help distinguish the type of implant [66, 67]. These slings work by dynamic compression [68], and the degree of ‘tightness’ can be inferred from sonographic appearances [69]. Ultrasound is useful in assessing women with complications such as voiding dysfunction or de novo symptoms of urgency and/or urge incontinence, helping the surgeon to decide whether to divide a sling, or remove part of the implant. Sling division usually results in a 5- to 10-mm gap between mesh arms which allows to document successful division.
Suburethral slings imaged on translabial ultrasound. In the midsagittal plane (a, c) the two slings are essentially indistinguishable as linear to curvilinear hyperechogenic structures. In the axial plane (b, d) the distinction is obvious: while the retropubic tape (b) is curving ventrally towards the symphysis pubis, the transobturator tape (d) arcs laterally towards the obturator foramen
Polypropylene mesh implants for prolapse surgery are not difficult to identify either, at least when placed in the anterior vaginal wall to treat cystocele. Figure 13 shows normal mesh appearances on Valsalva in the three orthogonal planes. The mesh is usually situated posterior to the bladder neck, caudal and dorsal to the trigone and the posterior bladder wall, and is apparent as a highly echogenic linear or curvilinear structure. It is more visible on Valsalva unless a rectocele or enterocele intervenes. Abnormally coarse, irregular appearances are commonly thought to be due to mesh shrinkage or contraction, but imaging data suggests that such appearances are likely due to excess mesh and/or surgical technique [70, 71], that is, folding and insufficient fixation to underlying tissues.
Identification of a transobturator mesh on Valsalva in the three orthogonal planes: midsagittal (a), coronal (b), and axial (c) Arrows show mesh extent in the midsagittal (left) and the coronal plane (center, right). S symphysis, B bladder, R rectum, L levator ani. The mesh is outlined with dots. From Dietz [70], with permission
Provided the current controversy surrounding mesh use does not preclude any chance of further development, translabial 4D ultrasound will be useful in determining functional outcome and location of implants, and will help in optimizing implant design and surgical technique. We have identified three distinct anatomical forms of failure after anterior compartment mesh use: (1) anterior failure: cystocele ventral and caudal to a well supported mesh; (2) apical failure: cystocele/anterior enterocele/uterine prolapse dorsal and caudal to a mesh with high mobility of the cranial mesh aspect; and finally (3) global failure: cystocele with high mobility of cranial and caudal mesh aspects on Valsalva. Most recurrent cystocele after mesh placement is due global or apical failures, suggesting dislodgment of lateral and/or apical anchors or fixation [72]. A small minority are anterior failures, implying dislodgment of the mesh from the bladder base due to faulty surgical technique.
Hopefully, the above illustrates the potential impact of functional imaging on postoperative audit and technological innovation and development in pelvic reconstructive surgery. Over the last decade, there has been a trend towards patient-centered outcomes in surgery, generally assessed using questionnaires. However, questionnaires are poor outcome measures for surgical trials as they are not very sensitive to change, and of course subjective outcomes allow no insight into pathophysiological mechanisms. Ultrasound may be a more powerful tool in assessing surgical outcomes than subjective symptoms and questionnaires [73]. Imaging measures of prolapse seem to be more strongly associated with symptoms rather than the results of a clinical assessment [74]. Hence, it seems reasonable to assume that sonographic assessment of surgical outcomes can enhance research and technological development in pelvic reconstructive surgery.
Axial Plane Imaging: The Levator Muscle and Hiatus
Direct imaging of the levator ani, which requires access to the axial plane, has experienced a boost with the development of 3D ultrasound. Side-firing vaginal transducers can image the axial plane on 2D, but they were never widely used. The translabial use of standard, commonly available abdominal 4D probes has advantages over endosonography, even if spatial resolutions are often inferior. With an acquisition angle of 70° or higher a single volume dataset includes the entire levator hiatus with symphysis pubis, urethra, paravaginal tissues, the vagina, anorectum, and puborectalis muscle. A Valsalva maneuver can result in displacement of parts of the puborectalis muscle outside the field of vision, especially in women with significant hiatal ballooning [75], which is why acquisition angles of 80° or even 85° are preferable.
Figure 3 demonstrates the main display modes on 3D/4D pelvic floor ultrasound imaging. The orthogonal display mode (A or midsagittal, B or coronal, C or axial plane) shows cross-sectional planes through the volume in question, with each plane situated at right angles to the other two. Distinct from MR, ultrasonic imaging planes can be varied arbitrarily to enhance the visibility of any structure. Imaging of the levator ani usually requires an axial plane that is slightly tilted, and this tilt varies substantially between patients and with maneuvers. Hence, it is very difficult to obtain correct axial planes on dynamic MR imaging, with a consequent reduction in accuracy [76].
The three orthogonal planes are often complemented by a ‘rendered image’, that is, a semitransparent representation of all pixels in a region of interest or ‘ROI’ defined by the investigator. Section d in Fig. 2 shows a ‘rendered image’, with the rendering direction set from caudally to cranially. The result is a representation that corresponds to seeing the patient’s pelvis from below. Rendering can substantially enhance the visibility of certain structures (e.g., synthetic implants) which helps patients and caregivers understand anatomical relationships. Figure 9 shows orthogonal and rendered volume representations of a typical true rectocele. 4D imaging involves the real-time acquisition of volume ultrasound data, that is, a succession of volumes over time, not just a single volume. This is particularly useful for the evaluation of functional anatomy, that is, for observing morphological changes during maneuvers (Valsalva or pelvic floor muscle contraction), which is virtually impossible on MR. And even if one was able to obtain true dynamic MR imaging, the lack of real-time control would preclude any opportunity for assessing the quality of such maneuvers and to avoid levator coactivation [19]. Hence, ultrasound has substantial advantages for prolapse assessment, especially when associated with fascial or muscular defects, and for defining functional anatomy. Finally, offline analysis software allow distance, area, angle, and volume measurements in any user-defined plane in a manner that is superior to what is currently possible with DICOM viewer software on MRI images.
Axial plane imaging is highly useful for the assessment of the levator ani muscle and hiatus, and for the examination of para-urethral tissues in patients with diverticula or strictures. Translabial ultrasound has confirmed forgotten 70-year-old clinical data [77] and recent MRI studies [78], showing that major structural abnormalities of the levator ani muscle are common in vaginally parous women [10] and that they are clearly due to vaginal childbirth [8]. Such defects are common and have now been confirmed by multiple research groups [12•, 79–82].
Figure 14 shows a comparison of clinical findings, ultrasound, and MR in a primiparous patient with recent unilateral levator avulsion after normal vaginal delivery. Such trauma can be identified by parasagittal 2D ultrasound [21]. However, the most reproducible approach is probably the use of 3D/4D abdominal probes developed for fetal imaging, placed translabially as shown in Fig. 1. Levator trauma can be diagnosed on rendered volumes [83], but multislice or tomographic imaging (as in Fig. 15) is likely to be more reproducible [84, 85•] and comparable to the diagnosis of levator trauma on MR [86••].
Delivery-related levator avulsion trauma as seen on exploration of a large vaginal tear after vaginal delivery (left), as imaged on translabial 4D ultrasound (middle), and on MR (right). (Reprinted from Dietz [109]; copyright 2009, with permission from the Australasian Society for Ultrasound in Medicine.)
Quantification of levator trauma on multislice/tomographic ultrasound imaging. There is a typical right-sided defect (indicated by asterisk). (Reprinted from Dietz [108], with permission from Springer.)
Major delivery-related levator injury clearly plays a substantial role in the aetiology of FPOP, although there are likely to be other birth-related factors, including ‘microtrauma’ or altered biomechanics of otherwise intact muscle [12•] and fascial trauma. Avulsion results in enlargement of the levator hiatus [87, 88] and is associated with reduced contractile strength [89]. It increases the likelihood of prolapse, especially in the anterior and central compartments [9, 10]. The larger the defect, the higher is the likelihood of prolapse [84], as quantified on multi-slice or tomographic ultrasound (see Fig. 15). Levator defects are also associated with cystocele recurrence after anterior repair with or without mesh [90••, 91•, 92]. These defects can be diagnosed clinically with palpation, which, however, does require significant teaching and is probably less repeatable than imaging [93].
Another factor that becomes fully apparent only in the axial plane is excessive distension (‘ballooning’) on Valsalva maneuver. Figure 16 gives an impression of the range of hiatal area measurements in patients attending a pelvic floor clinic in a tertiary hospital. The determination of hiatal dimensions seems to be highly repeatable [76, 94–96] and correlates well with findings on MRI [76]. Hiatal enlargement of ≥25 cm2 on Valsalva is defined as ‘ballooning’ on the basis of receiver operator characteristics statistics [75] and normative data in young nulliparous women [94]. It can be assessed both in axial plane slices at the plane of minimal hiatal dimensions and in rendered volumes, with the second method probably more valid due to the non-Euclidean (warped rather than flat) nature of the plane of minimal hiatal dimensions [97]. Ballooning can in fact be detected clinically by measuring the axial dimensions of the genital hiatus and perineal body on Valsalva [98•]. The degree of distension, both clinically and on ultrasound, is strongly associated with prolapse and symptoms of prolapse [75, 99, 100], and both avulsion and ‘ballooning’ seem to be independent risk factors of female POP [101].
Levator hiatal area (a normal narrow hiatus; b moderate ballooning in parous patient; c severe ballooning in patient with bilateral avulsion and 3 compartment prolapse) illustrating the range of findings that may be obtained in women with symptoms of lower urinary tract dysfunction. (Reprinted from Dietz [108], with permission from Springer.)
Currently, there is a highly controversial debate on mesh use. On the one hand, mesh implants have been shown to be effective in reducing prolapse recurrence [102••], especially in patients with levator avulsion [103]. On the other hand, mesh implants are associated with a higher rate of potentially serious complications such as chronic pain. Clearly, the balance between risk and benefit of mesh use will vary from one individual to the other; the real question is probably not whether to use mesh but in whom. Together with other published risk factors, such as young age, advanced prolapse stage before operation, family history of prolapse, previous failed surgery, enlarged urogenital hiatus, and Oxford grading [90••, 91•, 104, 105], the state of the patient’s pelvic floor may help identify patients at high risk of recurrence, justifying the consideration of mesh use.
Imaging may also allow the development of innovative surgical approaches that could obviate the need for intravaginal mesh use. Surgical attempts to reconnect the detached muscle both as a primary procedure in delivery suite [14] and as a secondary procedure during prolapse surgery [15] have been reported; however, results to date have been disappointing. In another development, surgical reduction of hiatal ballooning appears to be feasible [106] through accessing the ischiorectal fossa. The effect of hiatal reduction surgery on prolapse recurrence remains doubtful and will require a randomized controlled trial.
Conclusions
Sonographic imaging of pelvic floor structures, in particular in the form of translabial 3D/4D ultrasound, is becoming increasingly useful in the hands of physicians, surgeons, and researchers investigating pelvic floor disorders. The near-universal distribution of 4D ultrasound systems, new software options, and the increasing availability of training will likely lead to the acceptance of this method as an integral part of pelvic floor medicine. The issue of levator trauma, one of the most significant developments in clinical obstetrics in the last decade or two, will enhance this trend and enable an entirely new degree of cooperation and understanding between the different clinical specialties dealing with patients affected by pelvic floor disorders. As always, the provision of up-to-date resources for teaching and training of the next generation of healthcare providers is of paramount importance.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schubert E. Topographie des Uterus und der Harnblase im Roentgenprofilbild. ZentralblGynakol. 1929;53:1182–93.
Richter K. Die Bedeutung der radiologischen Beckenviszerographie fuer eine rationelle Therapie der weiblichen Stressinkontinenz. GeburtshilfeFrauenheilkd. 1987;47:509–17.
Kohorn EI, Scioscia AL, Jeanty P, Hobbins JC. Ultrasound cystourethrography by perineal scanning for the assessment of female stress urinary incontinence. Obstet Gynecol. 1986;68(2):269–72.
Grischke EM, Dietz HP, Jeanty P, Schmidt W. A new study method: the perineal scan in obstetrics and gynecology. Ultraschall Med. 1986;7(4):154–61.
Quinn MJ, Beynon J, Mortensen NJ, Smith PJ. Transvaginal endosonography: a new method to study the anatomy of the lower urinary tract in urinary stress incontinence. Br J Urol. 1988;62(5):414–8.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6.
Smith F, Holman D, Moorin R, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096–100.
Dietz H, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–12.
Dietz H, Simpson J. Levator trauma is associated with pelvic organ prolapse. Br J Obstet Gynaecol. 2008;115:979–84.
Dietz HP, Steensma AB. The prevalence of major abnormalities of the levator ani in urogynaecological patients. BJOG: Int J Obstet Gynaecol. 2006;113(2):225–30.
DeLancey J, Morgan D, Fenner D, Kearney R, Guire K, Miller J, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2):295–302.
• Shek K, Dietz H. Intrapartum risk factors of levator trauma. Br J Obstet Gynaecol 2010;117:1485–92. Largest cohort to date describing incidence of levator trauma and intrapartum risk factors.
• Shek K, Langer S, Chantarasorn V, Dietz H. Does the Epi-No device prevent levator trauma? A randomised controlled trial. Int Urogynecol J 2011;22(12):1521–8. First RCT aimed at prevention of pelvic floor trauma.
Dietz H, Gillespie A, Phadke P. Avulsion of the pubovisceral muscle associated with large vaginal tear after normal vaginal delivery at term. Aust NZ J Obstet Gynaecol. 2007;47:341–4.
Dietz H, Shek K, Daly O, Korda A. Can levator avulsion be repaired surgically? Int Urogynecol J. 2013;24:1011–5.
Dietz HP, Steensma AB. Posterior compartment prolapse on two- dimensional and three- dimensional pelvic floor ultrasound: the distinction between true rectocele, perineal hypermobility and enterocele. Ultrasound Obstet Gynecol. 2005;26:73–7.
Perniola G, Shek K, Chong C, Chew S, Cartmill J, Dietz H. Defecation proctography and translabial ultrasound in the investigation of defecatory disorders. Ultrasound Obstet Gynecol. 2008;31:567–71.
Dietz HP, Wilson PD. The influence of bladder volume on the position and mobility of the urethrovesical junction. Int Urogynecol J. 1999;10(1):3–6.
Oerno A, Dietz H. Levator co-activation is a significant confounder of pelvic organ descent on Valsalva maneuver. Ultrasound Obstet Gynecol. 2007;30:346–50.
Orejuela F, Shek K, Dietz H. The time factor in the assessment of prolapse and levator ballooning. Int Urogynecol J. 2012;23:175–8.
Dietz HP, Shek KL. Levator defects can be detected by 2D translabial ultrasound. Int Urogynecol J. 2009;20:807–11.
Koelbl H, Bernaschek G, Wolf G. A comparative study of perineal ultrasound scanning and urethrocystography in patients with genuine stress incontinence. Arch Gynecol Obstet. 1988;244(1):39–45.
Velez D, Shek K, Martin A, Dietz H. Determination of postvoid residual by translabial ultrasound. Int Urogynecol J. 2012;23(23):1749–52.
Tunn R, Petri E. Introital and transvaginal ultrasound as the main tool in the assessment of urogenital and pelvic floor dysfunction: an imaging panel and practical approach. Ultrasound Obstet Gynecol. 2003;22:205–13.
Dietz HP. Ultrasound imaging of the pelvic floor. Part I: two-dimensional aspects. [Review] [86 refs]. Ultrasound Obstet Gynecol. 2004;23(1):80–92.
Khullar V, Cardozo LD, Salvatore S, Hill S. Ultrasound: a noninvasive screening test for detrusor instability. Br J Obstet Gynaecol. 1996;103(9):904–8.
Lekskulchai O, Dietz H. Detrusor wall thickness as a test for detrusor overactivity in women. Ultrasound Obstet Gynecol. 2008;32:535–9.
Champaneria R, Latthe P, Khan K. Systematic review of the accuracy of ultrasound as the method of measuring bladder wall thickness in the diagnosis of detrusor overactivity. Int Urogynecol J. 2010;21:1019–24.
Salvatore S, Serati M, Cattoni E, Soligo M, Cromi A, Ghezzi F. Ultrasound measurement of bladder wall thickness in different forms of detrusor overactivity. Int Urogynecol J. 2010;21:1405–11.
Titus J, Blatt A, Chan L. Ultrasound measurment of bladder wall thickness in the assessment of voiding dysfunction. J Urol. 2008;179:2275–9.
Lekskulchai O, Dietz H. Is detrusor hypertrophy in women associated with symptoms and signs of voiding dysfunction? Aust NZ J Obstet Gynaecol. 2009;49:653–6.
Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultrasound for evaluating the bladder neck in urinary stress incontinence. Obstet Gynecol. 1995;85(2):220–4.
Dietz HP, Wilson PD. Anatomical assessment of the bladder outlet and proximal urethra using ultrasound and videocystourethrography. Int Urogynecol J. 1998;9(6):365–9.
Dietz H, Eldridge A, Grace M, Clarke B. Pelvic organ descent in young nulliparous women. Am J Obstet Gynecol. 2004;191:95–9.
Shek KL, Dietz HP. The urethral motion profile: a novel method to evaluate urethral support and mobility. Aust NZ J Obstet Gynaecol. 2008;48:337–42.
Pirpiris A, Shek K, Kay P, Dietz H. Urethral mobility and urinary incontinence. Ultrasound Obstet Gynecol. 2010;36:507–11.
Dickie K, Shek K, Dietz H. The relationship between urethral mobility and parity. Br J Obstet Gynaecol. 2010;117(10):1220–4.
Shek K, Kruger J, Dietz H. The effect of pregnancy on hiatal dimensions and urethral mobility: an observational study. Int Urogynecol J. 2012;23:1561–738.
Nazemian K, Shek K, Martin A, Dietz H. Can urodynamic stress incontinence be diagnosed by ultrasound? Int Urogynecol J 2013;24. doi:10.1007/s00192-012-2032-4.
Lewicky-Gaupp C, Blaivas JG, Clark A, McGuire EJ, Schaer G, Tumbarello J, et al. The cough game: are there characteristic urethtrovesical movement patterns associated with stress incontinence? Int Urogynecol J. 2009;20(2):171–5.
Dietz H. The aetiology of prolapse. Int Urogynecol J. 2008;19:1323–9.
Dietz H, Hansell N, Grace M, Eldridge A, Clarke B, Martin N. Bladder neck mobility is a heritable trait. Br J Obstet Gynaecol. 2005;112:334–9.
Dietz H, Clarke B. The prevalence of rectocele in young nulliparous women. Aust NZ J Obstet Gynaecol. 2005;45:391–4.
Dietz HP, Steensma AB. The role of childbirth in the aetiology of rectocele. Br J Obstet Gynaecol. 2006;113:264–7.
Dietz HP, Clarke B, Vancaillie TG. Vaginal childbirth and bladder neck mobility. Aust N Z J Obstet Gynaecol. 2002;42(5):522–5.
Dietz HP, Bennett MJ. The effect of childbirth on pelvic organ mobility. Obstet Gynecol. 2003;102(2):223–8.
•• Dietz HP. Pelvic Floor ultrasound in prolapse: what’s in it for the surgeon? Int Urogynecol J 2011;22:1221–32. Review of translabial ultrasound in the diagnosis of female pelvic organ prolapse.
Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164(2):428–33.
•• Dietz HP. Pelvic Floor ultrasound in incontinence: What’s in it for the surgeon? Int Urogynecol J 2011;22(9):1085–97. Review of translabial ultrasound in the diagnosis of female urinary incontinence.
•• Dietz HP, Beer-Gabel M. Ultrasound in the investigation of posterior compartment vaginal prolapse and obstructed defecation. Ultrasound Obstet Gynecol 2012;40:14–27. Review of translabial ultrasound in the diagnostic work-up of obstructed defecation.
Dietz HP. Can the rectovaginal septum be visualised on ultrasound? Ultrasound Obstet Gynecol. 2011;37(4):348–52.
Dietz HP, Korda A. Which bowel symptoms are most strongly associated with a true rectocele? Aust NZ J Obstet Gynaecol. 2005;45:505–8.
Rodrigo N, Shek K, Dietz H. Rectal intussusception is associated with abnormal levator structure and morphometry. Tech Coloproctol. 2011;15:39–43.
Beer-Gabel M, Teshler M, Barzilai N, Lurie Y, Malnick S, Bass D, et al. Dynamic transperineal ultrasound in the diagnosis of pelvic floor disorders: pilot study. Dis Colon Rectum. 2002;45(2):239–45.
Beer-Gabel M, Assoulin Y, Amitai M, Bardan E. A comparison of dynamic transperineal ultrasound (DTP-US) with dynamic evacuation proctography (DEP) in the diagnosis of cul de sac hernia (enterocele) in patients with evacuatory dysfunction. Int J Colorectal Dis. 2008;23:513–9.
Santoro GA, Wieczorek AP, Dietz HP, Mellgren A, Sultan A, Shobeiri SA, et al. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol. 2011;37:381–96.
• Steensma AB, Oom DMJ, Burger C, Schouten W. Assessment of posterior compartment prolapse: a comparison of evacuation proctography and 3D transperineal ultrasound.. Colorectal Dis 2010;12(6):533–9. Interesting comparative study of evacuation proctography and ultrasound in the assessment of patients with obstructed defecation.
Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993;36(4):976–83.
Peschers UM, DeLancey JO, Schaer GN, Schuessler B. Exoanal ultrasound of the anal sphincter: normal anatomy and sphincter defects. Br J Obstet Gynaecol. 1997;104(9):999–1003.
Guzman Rojas R, Shek K, Langer S, Dietz H. The prevalence of anal sphincter injury in primiparous women. Int Urogynecol J. 2012;23(S2):S226–8.
Yagel S, Valsky DV. Three-dimensional transperineal sonography for evaluation of the anal sphincter complex: another dimension in understanding peripartum sphincter trauma. Ultrasound Obstet Gynecol. 2006;27(2):119–23.
van de Geest L, Steensma AB. Three-dimensional transperineal ultrasound imaging of anal sphincter injuries after surgical primary repair. Ultrasound Obstet Gynecol. 2010;36(S1):270.
Weinstein M, Pretorius D, Jung S, Wan J, Nager C, Mittal RM. Anal sphincter complex muscles defects and dysfunction in asymptomatic parous women. Int Urogynecol J. 2011;22(9):1143–50.
Roos A, Abdool Z, Sultan A, Thakar R. The diagnostic accuracy of endovaginal and transperineal ultrasound for detecting anal sphincter defects: the PREDICT study. Clin Radiol. 2011;66(7):597–604.
Shek K, Guzman Rojas R, Dietz HP. Residual defects of the external anal sphincter are common after OASIS repair. Neurourol Urodyn. 2012;31(6):913–4.
Dietz HP, Barry C, Lim YN, Rane A. Two-dimensional and three-dimensional ultrasound imaging of suburethral slings. Ultrasound Obstet Gynecol. 2005;26(2):175–9.
Dietz H, Barry C, Lim Y, Rane A. TVT vs monarc: a comparative study. Int Urogynecol J. 2006;17:566–9.
Dietz H, Wilson P. The Iris Effect: how 2D and 3D volume ultrasound can help us understand anti-incontinence procedures. Ultrasound Obstet Gynecol. 2004;23:267–71.
Chantarasorn V, Shek K, Dietz H. Sonographic appearance of transobturator slings: implications for function and dysfunction. Int Urogynecol J. 2011;22:493–8.
Dietz H, Erdmann M, Shek K. Mesh contraction: myth or reality? Am J Obstet Gynecol. 2011;204(2):173.e1–4.
Svabik K, Martan A, Masata J, El Haddad R, Hubka P. Ultrasound appearances after mesh implantation-evidence of mesh contraction or folding? Int Urogynecol J. 2011;22(5):529–33.
Shek K, Wong V, Rane A, Goh J, Krause H, Lee J, et al. How common is fixation failure after mesh kit surgery? Int Urogynecol J. 2012;23(S2):S154–5.
Svabik K, Haddad R, Masata J, Hubka P, Martan A. Does the grade of vaginal wall prolapse corrrelate with the pelvic organ prolapse detress inventory (POPDI) scores? Int Urogynecol J. 2012;23:S209.
Endress E, Shek K, Dietz H. Can we distinguish between congenital and traumatic prolapse? Anstract, IUGA Brisbane; 2012.
Dietz H, De Leon J, Shek K. Ballooning of the levator hiatus. Ultrasound Obstet Gynecol. 2008;31:676–80.
Kruger J, Heap X, Murphy B, Dietz H. Pelvic floor function in nulliparous women using 3-dimensional ultrasound and magnetic resonance imaging. Obstet Gynecol. 2008;111:631–8.
Gainey HL. Post-partum observation of pelvic tissue damage. Am J Obstet Gynecol. 1943;46:457–66.
DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101(1):46–53.
Valsky DV, Lipschuetz M, Bord A, Eldar I, Messing B, Hochner-Celnikier D, et al. Fetal head circumference and length of second stage of labor are risk factors for levator ani muscle injury, diagnosed by 3-dimensional transperineal ultrasound in primiparous women. Am J Obstet Gynecol. 2009;201:91.e1–7.
Albrich S, Laterza R, Skala C, Salvatore S, Koelbl H, Naumann G. Impact of mode of delivery on levator morphology: a prospective observational study with 3D ultrasound early in the postpartum period. Br J Obstet Gynaecol. 2012;119(1):51–61.
Chan S, Cheung R, Yiu A, Lee L, Pang A, Choy K, et al. Prevalence of levator ani muscle injury in Chinese primiparous women after first delivery. Ultrasound Obstet Gynecol. 2012;39(6):704–9.
Eisenberg V, Brecher S, Yodfat I, Bitman G, Achiron R, Schiff E, et al. Risk factors for levator avulsion trauma in women with obstetric anal sphincter injuries. Ultrasound Obstet Gynecol. 2011;38(S1):52.
Dietz H, Moegni F, Shek K. Diagnosis of levator avulsion injury: a comparison of three methods. Ultrasound Obstet Gynecol. 2012;40(6):693–8.
Dietz H. Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet Gynecol. 2007;29:329–34.
• Dietz H, Bernardo M, Kirby A, Shek K. Minimal criteria for the diagnosis of avulsion of the puborectalis muscle by tomographic ultrasound. Int Urogynecol J 2011;22(6):699–704. Defines the currently optimal method for diagnosing levator trauma.
•• Zhuang R, Song Y, Chen Q, Ma M, Huang H, Chen J, et al. Levator avulsion using a tomographic ultrasound and magnetic resonance-based model. Am J Obstet Gynecol 2011;205:232.e1–8. Compares MRI and transperineal tomographic ultrasound in the diagnosis of levator trauma.
Abdool Z, Shek K, Dietz H. The effect of levator avulsion on hiatal dimensions and function. Am J Obstet Gynecol. 2009;201:89.e1–5.
Shek K, Dietz H. The effect of childbirth on hiatal dimensions: a prospective observational study. Obstet Gynecol. 2009;113:1272–8.
Dietz HP, Shek C. Levator avulsion and grading of pelvic floor muscle strength. Int Urogynecol J. 2008;19(5):633–6.
•• Dietz HP, Chantarasorn V, Shek KL. Levator avulsion is a risk factor for cystocele recurrence. Ultrasound Obstet Gynecol 2010;36:76–80. First paper demonstrating that levator trauma is a risk factor for recurrence of female pelvic organ prolapse after reconstructive surgery.
• Weemhoff M, Vergeldt T, Notten K, Serroyen J, Kampschoer P, Roumen F. Avulsion of puborectalis muscle and other risk factors for cystocele recurrence: a 2-year follow-up study. Int Urogynecol J 2012;23(1):65–71. Comprehensive investigation of potential risk factors for recurrence of female pelvic organ prolapse after reconstructive surgery.
Wong V, Shek K, Goh J, Rane A, Dietz HP. Is levator avulsion a predictor for cystocele recurrence following anterior compartment mesh? Neurourol Urodyn. 2011;30(6):879–80.
Dietz HP, Shek KL. Validity and reproducibility of the digital detection of levator trauma. Int Urogynecol J. 2008;19:1097–101.
Dietz H, Shek K, Clarke B. Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet Gynecol. 2005;25:580–5.
Yang J, Yang S, Huang W. Biometry of the pubovisceral muscle and levator hiatus in nulliparous Chinese women. Ultrsound Obstet Gynecol. 2006;26:710–6.
Hoff Braekken I, Majida M, Ellstrom Engh M, Dietz HP, Umek W, Bo K. Test- retest and intra-observer repeatability of two-, three- and four- dimensional perineal ultrasound of pelvic floor muscle anatomy and function. Int Urogynecol J. 2008;19:227–35.
Dietz H, Wong V, Shek KL. A simplified method for determining hiatal biometry. Aust NZ J Obstet Gynaecol. 2011;51:540–3.
• Khunda A, Shek K, Dietz H. Can ballooning of the levator hiatus be determined clinically? Am J Obstet Gynecol 2012;206(3):246.e1–4. First paper describing the clinical assessment of hiatal dimensions.
Dietz H, Steensma A. Dimensions of the levator hiatus in symptomatic women. Ultrasound Obstet Gynecol. 2005;26(4):369–70.
Gerges B, Kamisan Atan I, Shek K, Dietz H. How to determine ‘ballooning’ of the levator hiatus on clinical examination. Int Urogynecol J. 2012;23(S2):S52–3.
Rodrigo N, Shek K, Wong V, Martin A, Dietz H. Hiatal ballooning is an independent risk factor of prolapse recurrence. Int Urogynecol J. 2012;23(S2):S 129–30.
•• Altman D, Väyrynen T, Ellström Engh M, Axelsen S, Falconer C. Anterior Colporrhaphy versus Transvaginal Mesh for Pelvic-Organ Prolapse. New Engl J Med 2011;364:1826–36. First large RCT demonstrating benefits of mesh placement in cystocele repair.
Wong V, Shek K, Goh J, Rane A, Dietz H. Should mesh be used for cystocele repair? Long- term outcomes of a case- control series. Int Urogynecol J. 2011;22(S1):S91.
Vakili B, Zheng Y, Loesch H, Echols K, Franco N, Chesson RR. Levator contraction strength and genital hiatus as risk factors for recurrent pelvic organ prolapse. Am J Obstet Gynecol. 2005;192:1592–8.
Morgan D, Larson K, Lewicky-Gaupp C, Fenner D, DeLancey J. Vaginal support as determined by levator ani defect status 6 weeks after primary surgery for pelvic organ prolapse. Int J Gynaecol Obstst. 2011;114(2):141–4.
Dietz H, Korda A, Benness C, Wong V, Shek K, Daly O. Surgical reduction of the levator hiatus. Neurourol Urodyn. 2012;31(6):872–3.
Dietz HP. The role of two- and three-dimensional dynamic ultrasonography in pelvic organ prolapse. J Minim Invasive Gynecol. 2010;17:282–94.
Dietz HP. Pelvic floor ultrasound, chapter 4.5. In: Stoker J, Taylor SA, De Lancey JOL, editors. Imaging pelvic floor disorders. London: Springer Verlag; 2008.
Dietz HP. Pelvic floor ultrasound. ASUM Ultrasound Bull. 2007;10:17–23.
Dietz HP. Pelvic floor ultrasound. In: Fleischer AC, Toy EC, Wesley L, Manning FA, Romero R, editors. Sonography in obstetrics and gynaecology: principles and practice, 7th ed. New York: McGraw Hill; 2011.
Diclosure
Hans Peter Dietz has served as a consultant for Materna and has received grant support and had travel/accommodation expenses covered/reimbursed by GE Medical.
Compliance with Ethics Guidelines
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dietz, H.P. Pelvic Floor Ultrasound. Curr Surg Rep 1, 167–181 (2013). https://doi.org/10.1007/s40137-013-0026-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40137-013-0026-x