Abstract
Introduction
Admissions for acute bacterial skin and skin structure infections (ABSSSI) are often prolonged because of intravenous (IV) antibiotics. Use of a long-acting IV antibiotic may reduce length of stay (LOS) on a hospitalist service. The ENHANCE ABSSSI trial sought to determine the impact on LOS and work productivity in patients treated with a long-acting IV antibiotic, dalbavancin, vs. usual care at an urban tertiary-care center.
Methods
A single-center, pre- vs. post-period pragmatic trial at Weill-Cornell Medical Center assessed usual care for consecutively enrolled admitted ABSSSI patients during an observational period (pre-period). Identification and treatment of eligible admitted ABSSSI patients with dalbavancin were implemented in the post-period. Those with life-threatening infections, requiring multiple antibiotics/intensive care, or with unstable comorbidities were excluded. Outcomes were assessed over a 44-day follow-up period.
Results
Of 48 and 43 patients enrolled, respectively, in the pre- and post-periods, mean infection-related LOS was reduced in the post-period (3.2 days vs. 4.8 days; P = 0.003). Similar results were found in an adjusted LOS analysis. Work productivity and activity impairment outcomes significantly improved in the post-period (P ≤ 0.01). Complete response rates were similar: 50% (pre-period) and 57% (post-period). Among AEs identified, 17% (n = 7) were found to have possible causal relation to dalbavancin in the post-period. Few AEs were serious (n = 3; 7% post-period versus n = 1; 2% pre-period).
Conclusion
After implementing the ENHANCE ABSSSI pathway, LOS was significantly reduced by almost 2 days, with potential improvements in work productivity and ability to complete daily activities.
Trial Registration
ClinicalTrials.gov identifier, NCT03233438.
Funding
Allergan plc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Admissions for acute bacterial skin and skin structure infections (ABSSSI) are often prolonged because of administration of intravenous (IV) antibiotics |
Use of a long-acting IV antibiotic, dalbavancin, may reduce length of stay (LOS) on a hospitalist service when compared to usual care |
What was learned from the study? |
Determined the impact on LOS and work productivity in ABSSSI patients treated with long-acting IV antibiotic therapy vs. usual care at an urban tertiary-care center |
After implementing the ENHANCE ABSSSI pathway, LOS was significantly reduced by almost 2 days and improved patient’s work productivity and the ability to complete daily activities |
Implementation of the ENHANCE ABSSSI pathway could have a significant impact on hospital cost and efficiency |
Introduction
Quality of care and efficiency are paramount to improving US healthcare systems, particularly in the high-cost inpatient setting where hospitalists practice. Hospital admissions are under evaluation through a number of measures and must be critically assessed for improvement [1]. Care for acute bacterial skin and skin structure infections (ABSSSIs) has traditionally involved routine hospital admission and potentially prolonged hospital length of stay (LOS). The primary reason for hospitalization is the need to receive intravenous (IV) antibiotic therapy [2], despite the majority of ABSSSIs being non-life-threatening compared with other infections [3, 4]. ABSSSIs comprise as much as 2% of all US hospital admissions, causing a substantial burden to US hospitals, largely due to room and board costs [5]. ABSSSIs also commonly occur among employed adults (18–44 years of age) [5,6,7]; patients would value the option to complete ABSSSI treatment at home to avoid interference with daily activities and work [8].
Current guidelines support shifting ABSSSI care to outpatient settings for appropriate patients to improve hospital efficiency and reduce cost [4, 9,10,11,12]. However, operational barriers exist to shifting care to outpatient settings for many hospitals in the emergency department (ED). Implementation may suffer from lack of feasibility in care coordination between ED physicians and Antimicrobial Stewardship Teams before admission or because of high patient flow through many EDs.
For certain hospitals, ABSSSI treatment with a long-acting antibiotic once patients are admitted to the hospital floor and are stabilized is a potential option to facilitate discharge. Among those admitted for ABSSSI, those who may be eligible for early discharge would have few risk factors for readmission or ED care [4, 9, 11,12,13]. Careful patient selection is necessary to support efficiency and to ensure that treatment outcomes are similar versus standard care.
We reasoned that a long-acting lipoglycopeptide may be utilized to facilitate discharge for eligible patients because of an abbreviated administration schedule [9, 12, 14], allowing patients to finish their treatment course in the outpatient setting [4, 10]. Long-acting antibiotics, such as dalbavancin, are administered either as a single 30-min parenteral infusion or two 30-min infusions once a week for 2 weeks [15, 16] or, in the case of oritavancin, as a 3-h infusion [17]. Long-acting antibiotics may improve patient and health system convenience as well as reduce risks of infection from the peripheral or central catheter lines that are used for some patients to receive daily parenteral antibiotics. Additionally, single-dose antibiotic treatment of ABSSSI is aligned with patient preferences [8], which are increasingly important as patient satisfaction is included in CMS hospital quality measures [18].
ENHANCE (A Pragmatic Trial Designed to Evaluate a New Critical Pathway for Treatment of Patients with ABSSSI) was a controlled trial that sought to determine the impact on LOS and work productivity in patients treated with the long-acting IV antibiotic, dalbavancin, vs. usual care at an urban, tertiary care center.
Methods
Study Design and Setting
ENHANCE was a single-center, pre- versus post-period pragmatic trial (NCT03233438) conducted at New York-Presbyterian/Weill Cornell Medicine (NYP/WCM), a university-based urban medical center with > 1000 hospital beds, almost 50,000 hospital admissions, and nearly 75,000 ED visits per year. At the start of the trial, the site did not have dalbavancin routinely available.
The ENHANCE study design consisted of an initial observational period (pre-period); second, an interventional period (post-period) was subsequently implemented. In the pre-period only, treating physicians were not included in the study enrollment decision or trained on the protocol to prevent biasing the provision of usual care, which was defined as site or physician’s usual care for ABSSSI. Once the pre-period was completed, site-training materials were provided to the study team on the post-period of the study. Post-period training materials were based on the study protocol and prior literature [19]. Over a 2–4 week period, principal site investigators (MM and TW) trained treating physicians and staff; training was documented before beginning the post-period of the study. During the post-period, a separate set of enrolled patients was treated, which included (1) ABSSSI patient identification (inclusion/exclusion criteria applied) and (2) long-acting antibiotic treatment, administered as a single intravenous (IV) dose of 1500 mg dalbavancin over 30 min; patients with renal impairment (creatinine clearance < 30 ml/min) and no regularly scheduled dialysis received a single IV dose of 1125 mg dalbavancin over 30 min [15]. All patients enrolled in the post-period received dalbavancin. At the discretion of the treating physicians, patients were discharged from the hospital to an outpatient setting after receipt of dalbavancin. While randomization at the site level at NYP/WCM was not feasible, the study was controlled temporally through the pre- vs. post-period design [20,21,22,23].
The study was conducted in accordance with the protocol, rules, and regulations of the WCM IRB and standard operating procedures, and sponsor at WCM, and informed consent regulations. Only patients providing informed consent were included in the study. The study followed the hospital’s applicable local regulatory requirements, was governed according to local laws and regulations, and was in accordance with the Declaration of Helsinki.
Study Patients
Adult patients (≥ 18 years) admitted to the NYP/WCM hospitalist service with an ABSSSI infection (e.g., diagnosis of cellulitis/erysipelas, wound infection, or major cutaneous abscess) [4, 10, 24] were identified and enrolled based on inclusion and exclusion criteria through patient interview and medical chart review. Enrollment criteria were based on ABSSSI treatment guidelines regarding patients for whom parenteral therapy was indicated because of infection severity, yet could otherwise receive treatment in an outpatient setting [4, 11]. Those with life-threatening infections, requiring multiple antibiotics or intensive care, or with unstable comorbidities were not eligible (Supplementary Text: Inclusion/Exclusion Criteria).
Follow-up and Outcomes
Patients were followed for 44 days after study enrollment to account for the first 10–14 days of initial ABSSSI treatment and for 30 days after treatment; baseline characteristics (Supplementary Text: Study Definitions), health resource utilization, patient-reported outcomes, response to treatment, and adverse events (AEs) were captured (Table S1). Baseline characteristics were collected before patient enrollment in the study at the screening visit and were based on physical examination, patient interview, and medical chart review. Follow-up visits were scheduled between 48 and 72 h after hospital discharge, 10–14 days after enrollment (end of treatment visit), and 44 days after enrollment. Health resource utilization through patient interviews and medical chart review as well as patient-reported outcomes was captured through follow-up visits at 10–14 days and 44 days. Response to treatment was determined by patient interview and examination at 48–72 h and visits at 10–14 days. Safety monitoring was conducted at each visit through the 44-day follow-up period.
Baseline characteristics included demographics, comorbidities, Charlson Comorbidity Index, infection type, infection and clinical characteristics, and utilization of the healthcare service within the past 3 months. The primary outcome was infection-related LOS. Secondary outcomes included other healthcare resource utilization during follow-up, such as all-cause LOS, all-cause 30-day re-admissions, ED visits, and outpatient visits, patient-reported outcomes, response to treatment, and AEs. Patient-reported outcomes including quality of life and work productivity were collected through validated questionnaires (Health-Related Quality-of-Life Medical Outcomes Study Short Form-12 [SF-12] [25], Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0 [WPAI:SHP]) [26]. Patient satisfaction with care was captured using a descriptive survey developed for use in this study. These questionnaires are included in Supplementary Text: Patient Satisfaction Survey.
Statistical Methods
We hypothesized that administration of dalbavancin, a long-acting lipoglycopeptide, in the post-period would decrease LOS and facilitate discharge for eligible patients vs. patients who received usual care in the pre-period. Sample size was determined through a power calculation; with 80% power, a sample size of 86 patients (43 patients per period) supported a decrease in LOS of 2 days in the post-period vs. the pre-period. Outcomes were calculated for the analysis sample, which was defined as all enrolled patients who received ≥ 1 antibiotic dose in the pre-period or one dose of dalbavancin in the post-period. Descriptive statistics, including continuous and categorical variables, were reported for all outcomes. Two-sided Student’s t tests were used for applicable continuous variables, and Mann-Whitney U tests were used if the event normality assumptions were not met. For categorical variables, periods were compared using Chi-squared tests or Fisher’s exact tests, as appropriate. All statistical testing applied a significance level of 0.05.
Primary LOS outcome was compared between periods through a two-sided t test. To account for baseline differences in characteristics between periods, a generalized linear mixed model was also used in an adjusted analysis using a negative binomial distribution with a log link. The final multivariable model was created through stepwise selection and a statistical significance threshold of α < 0.1 for clinically relevant variables specified a priori (Table S2). The final model was adjusted for age and immunocompromised status.
Results
Patient Demographics and Disease Characteristics
Among 112 patients screened, 48 patients were enrolled in the pre-period (July 2017–November 2017). During the post-period, among 131 patients screened, 43 patients were enrolled in the post-period from February 2018 to September 2018 (Fig. 1). The attrition rate was 12.5% and 23.3% in the pre- and post-periods, respectively, where loss to follow-up was the primary reason for study withdrawal for both periods. From the enrolled set, one patient from the post-period was not included in the analysis sample as they did not receive ≥ 1 dose of antibiotics after enrollment; therefore, 48 patients in the pre-period and 42 patients in the post-period were included in the analysis of study outcomes.
Patient disposition. *Patient 2010047 provided informed consent for the study, but withdrew it shortly thereafter. Due to timing of consent withdrawal, the patient was removed from the study database. EC3: Known or suspected infections that are severe, life-threatening, or not included in the ABSSSI FDA guidance, including the following examples: gangrene; known or suspected necrotizing fasciitis; known or suspected osteomyelitis, septic arthritis, or endocarditis; diabetic foot infection; and decubitus or ischemic ulcer. EC9: Unwilling or unable to follow study procedures. IC3: Willing and able to return to the hospital or a designated clinic for scheduled visits, or be in contact with the study coordinator through telephone communication, as required by the protocol and the antibiotic treatment administered
Demographics and baseline characteristics were largely similar between groups (Table 1). The majority of patients were admitted from a private home or an independent living facility in the pre-period (n = 48; 100%) and post-period (n = 36; 85.7%). More patients in the pre-period were unemployed (83%) vs. post-period (52%).
Disease characteristics and medical history were similar between groups (Table 1). Systemic inflammatory response syndrome criteria were met in 11 (22.9%) patients of the pre-period and 8 (19.0%) patients of the post-period. The mean (standard deviation)/median primary lesion size in the pre-period was 533.5 (647.9)/255 cm2 and in the post-period was 685.0 (703.5)/458 cm2. One patient in the pre-period and two patients in the post-period had evidence of a recurrent ABSSSI infection in the prior 6 months.
Antibiotic Treatment Received
In the pre-period group, most patients (66.7%) received multiple antibiotic therapies, with 17.8% of patients receiving > 4 antibiotics. In contrast, most patients (85.7%) received one antibiotic (i.e., dalbavancin) in the post-period. Oral (85.4%) and IV (58.3%) antibiotics were used in the pre-period group, while IV antibiotics were the predominant administration route in the post-period group (100%). Among patients with a reported oral administration route, the majority (64.4%) received oral and IV antibiotics. Moreover, among the pre-period group, 87.5% of patients completed their initial antibiotic treatments compared with 100% in the post-period group.
Effects on LOS
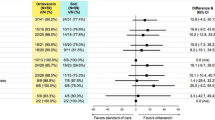
A significant reduction in mean infection-related LOS was supported in the post- vs. pre-period (3.2 days vs. 4.8 days; P = 0.003) (Table 2; Fig. 2). All-cause LOS was also significantly reduced (3.2 days vs. 4.9 days; P = 0.003). Similar results were found after infection-related LOS was adjusted for age and immunocompromised status (3.9 days vs. 5.5 days; P = 0.002).
Half of all patients from the post-period group (21 patients, 50%) were discharged to complete care in an outpatient setting on the same day dalbavancin was provided. Of the 50% who were not discharged on the same day, the most common reasons were due to additional monitoring/procedures for ABSSSI (10 patients; 47.6%) or non-ABSSSI reasons (9 patients; 42.9%).
Effect on Other Health Resource Utilization
No major surgical interventions occurred during pre-period follow-up; one was identified during post-period follow-up for non-ABSSSI care (breast reconstruction). All-cause hospitalization in the 30 days after discharge was numerically higher in the post- vs. the pre-period (n = 3 vs. n = 1). During follow-up, no patient from the pre-period group and two patients from the post-period group were re-hospitalized because of the index ABSSSI. Over the entire study duration, no infection-related hospitalization resulted in admission to ICU in either group (Table 3).
Effects on Patient-reported Outcomes
Although fewer than half of the patients responded, the work productivity and activity impairment (impairment while working, overall work impairment, and non-work related impairment of activity) outcomes significantly improved in the post-period (P ≤ 0.01) (Table 4). Absenteeism and quality of life outcomes were not significantly different between the pre- and post-periods.
A smaller proportion of patients in the pre-period group expressed no concern about receiving an IV therapy compared with patients in the post-period group (Table 5; 54.8% vs. 78.8%). If they were treated again for a similar ABSSSI, patients from both groups preferred (65.1% and 97.1%, respectively) receiving one single dose of an IV antibiotic. Most patients would find value in a physician who recommended that they receive an IV antibiotic therapy that might shorten their hospital LOS.
Therapeutic Response
The percentage of patients with complete treatment response was similar between periods (50.0% in the pre-period and 57.1% in the post-period; Table 4).
Adverse Events
Among AEs identified, 16.7% (n = 7) were found to have a possible causal relation to a study drug in the post-period. A greater proportion of AEs occurred in the post-period (n = 20; 47.6%) vs. pre-period (n = 3; 6.3%) with most AEs being mild in severity and not related to antibiotic use; few AEs were serious (7.1% post-period vs. 2.1% pre-period) (Table 4). One of the serious AEs (vomiting) was considered to be probably related to the study drug; it was mild in severity and occurred 2 h after dalbavancin treatment for a patient in the post-period. The most common AEs were unrelated infection in the pre-period and fever in the post-period.
Discussion
As hospitals are under pressure to improve quality of care, a critical evaluation of current pathways and processes is warranted. Admission to the hospitalist service for ABSSSI imposes a significant economic burden on the US health system, with hospital LOS as a key cost driver [5, 6, 12, 27,28,29]. We found that LOS was ~ 5 days on average in our study for those patients in the pre-period vs. the post-period LOS of ~ 3 days. Due to the increasing emphasis on efficiency and quality of care, the development of new ABSSSI care pathways that improve hospital efficiency and reduce the costs associated with unnecessary or prolonged hospital admissions is warranted [4, 9,10,11,12]. A key component of a new care pathway may leverage long-acting antibiotic therapy, such as dalbavancin. Yet, it is necessary to not only support process improvements, but also to establish the safety and efficacy of new care pathways in a real-world setting.
Our study is the first pragmatic clinical trial to date to implement use of a long-acting antibiotic, dalbavancin, for ABSSSI treatment among admitted patients on a hospitalist service in a large hospital setting. Following implementation of dalbavancin treatment in this real-world study, infection-related hospital LOS and overall LOS were significantly decreased by nearly 2 days. Additional health resource utilization, indicative of poor outcomes or the need for follow-up care, such as 30-day hospitalization rates, use of the intensive care unit, and ED and outpatient visits, was found to be similar between periods. As patient satisfaction is of increasing importance in measuring quality of care [18], patient-reported work or activity impairment were also significantly reduced in patients in the post- vs. pre-period. Patients in the post-period reported similar health related quality of life and patient satisfaction. Our results were similar to prior patient preference research [8] in that the majority of patients preferred antibiotic regimens similar to dalbavancin (i.e., single dose, shorter infusion durations) over other options, and most would find value in a physician who provided an option that could shorten their hospital LOS. This latter option is likely linked to the improved patient-reported indices of work productivity. It should be noted, however, that there was a higher attrition rate and a higher percentage of patients readmitted to the hospital in the post-period. These cases included individuals with concomitant medical conditions requiring further care and did not represent antimicrobial treatment failure for ABSSSI.
In addition to increasing quality and efficiency of care, ensuring the response to treatment and safety profile of the dalbavancin pathway is paramount. Response to treatment was similar between the treatment groups. As a more clinically meaningful measure of safety versus AEs overall, 16.7% (n = 7) of AEs were found to have a possible causal relation to dalbavancin in the post-period. The number of AEs in patients within the post-period were higher vs. the pre-period, while serious AEs were similar between periods. Moreover, no patients discontinued treatment, and mortality did not occur in either period. The AEs were largely unrelated to the infection being treated or due to known toxicities of dalbavancin [30]. The more involved informed consent process in the post-period versus the pre-period (observational design) may have contributed to difference in AEs between periods. These measures support our hypothesis that ENHANCE may be an effective alternative care pathway.
Our research builds on prior evidence that dalbavancin is a safe, effective [30,31,32], and potentially cost-saving option for outpatient ABSSSI treatment [33]. Outpatient treatment has been shown to result in reduced hospital stay resulting in lower costs [5, 12, 27, 29], highlighting the importance of alternative treatment options. Indeed, the estimated cost of a day in a non-profit hospital located in New York (similar to the characteristics of NYP/WCM) is approximately $2719 [34]. Considering LOS for ENHANCE post-period patients was reduced by 1.6 days, the estimated savings would partially or fully offset the cost of long-acting antibiotic therapy, such as dalbavancin. Such an estimate does not take into account the costs of pharmacy and nursing for administration.
Despite the higher acquisition cost of dalbavancin one could hypothesize that the reduced LOS, increased work activity, and reduction in the use of multiple inpatient antibiotics could lead to significant cost savings. We also suggest that a registry of the use of dalbavancin be implemented in those institutions adapting the ENHANCE ABSSSI pathway to prospectively monitor the impact of this strategy.
Although a potential limitation of the study might be the single-center design, this design feature eliminated variability that could occur in a study involving multiple hospitals, such as number of hospital staff, staffing hours, etc. However, it may limit the generalizability of our findings. A prospective multicenter registry could be implemented to validate this approach to management of patients hospitalized with ABSSSI. Another limitation was lack of randomization in this study, which could bias results because of differences between pre- and post-period patients at baseline. Yet, our study was consistent with real-world clinical practices, as randomization of patients or hospitals to receive different clinical pathways is not always feasible, and we found similar baseline characteristics between both periods [23]. Consistent with other studies, we observed a seasonality to ABSSSI (i.e., infections were more prevalent during the summer months). Although randomization at the level of the patient or site was not implemented, the pre-period of the study served as an observational control group, supporting a controlled trial design. We acknowledge that selection bias is an important factor that can impact study design; however, we believe that selection criteria were similarly applied in each study period through consistently implemented site training.
Conclusion
In summary, the ENHANCE ABSSSI pathway among eligible patients on a hospitalist service significantly reduced LOS by 1.6 days with improvements in work productivity and the ability to complete daily activities.
References
Centers for Medicare & Medicaid Services. CMS Quality Strategy. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Quality-Strategy.pdf. Accessed 27 June 2019.
Talan DA, Salhi BA, Moran GJ, et al. Factors associated with decision to hospitalize emergency department patients with skin and soft tissue infection. West J Emerg Med. 2015;16:89–97.
Gunderson CG, Cherry B, Fisher A. Mortality of hospitalized patients with cellulitis: a systematic review and meta-analysis. In: Presented at: Hospital Medicine, April 8–11, 2018; Orlando, FL.
Pollack CV Jr, Amin A, Ford WT Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med. 2015;48:508–19.
Keyloun KR, Weber DJ, Gardstein BM, et al. Economic burden of hospital admissions for patients with acute bacterial skin and skin structure infections in the United States. Hosp Pract. 1995;2018(46):278–86.
Kaye KS, Patel DA, Stephens JM, et al. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One. 2015;10:e0143276.
McDermott KW, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005-2014, Statistical brief #225. Healthcare Cost and Utilization Project (HCUP). Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.jsp. Accessed 27 June 2019.
Almarzoky Abuhussain SS, Burak MA, Kohman KN, et al. Patient preferences for treatment of acute bacterial skin and skin structure infections in the emergency department. BMC Health Serv Res. 2018;18:932.
Bosso JA, Casapao AM, Edwards J, et al. Clinical pathway for moderate to severe acute bacterial skin and skin structure infections from a US perspective: a roundtable discussion. Hosp Pract. 1995;2016(44):183–9.
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10–52.
Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–72.
Jensen IS, Lodise TP, Fan W, et al. Use of oritavancin in acute bacterial skin and skin structure infections patients receiving intravenous antibiotics: a US hospital budget impact analysis. Clin Drug Investig. 2016;36:157–68.
Stenehjem E, Brown J, Goldwater S, Scoble P, Owens R, Jr. Factors driving emergency department revisits or hospitalization within 30 days post-discharge (DC) in patients with acute bacterial skin and skin structure infections (ABSSSI). In: Presented at: IDWeek October 7–11, 2015; San Diego, CA.
Lane S, Johnston K, Sulham KA, et al. Identification of patient characteristics influencing setting of care decisions for patients with acute bacterial skin and skin structure infections: results of a discrete choice experiment. Clin Ther. 2016;38:531–44 (quiz 44 e1-9).
Dalvance® (dalbavancin). Full prescribing information. Parsippany: Durata Therapeutics US Ltd.; 2018.
Garnock-Jones KP. Single-dose dalbavancin: a review in acute bacterial skin and skin structure infections. Drugs. 2017;77:75–83.
Orbactiv® (oritavancin). Full prescribing information. Lincolnshire: Melinta Therapeutics, Inc; 2019.
Centers for Medicare & Medicaid Services. HCAHPS: Patients’ Perspectives of Care Survey. Available at: www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalHCAHPS.html. Accessed 27 June 2019.
Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283:749–55.
Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb). 2014;24:199–210.
Harris AD, McGregor JC, Perencevich EN, et al. The use and interpretation of quasi-experimental studies in medical informatics. J Am Med Inf Assoc. 2006;13:16–23.
Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–63.
Macpherson H. Pragmatic clinical trials. Complement Ther Med. 2004;12:136–40.
US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. https://www.fda.gov/files/Acute-Bacterial-Skin-and-Skin-Structure-Infections—Developing-Drugs-for-Treatment.pdf. Accessed 15 May 2019.
Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33.
Reilly Associates. WPAI:SHP V2.0. http://www.reillyassociates.net/WPAI_SHP.html. Accessed 21 Oct 2019
Ektare V, Khachatryan A, Xue M, et al. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18:1092–101.
LaPensee KT, Fan W. Economic burden of hospitalization with antibiotic treatment for bacteremia/sepsis in the US. In: Presented at: IDWeek Annual Meeting, October 17–21, 2012; San Diego, CA.
Lodise TP, Fan W, Sulham KA. Economic impact of oritavancin for the treatment of acute bacterial skin and skin structure infections in the emergency department or observation setting: cost savings associated with avoidable hospitalizations. Clin Ther. 2016;38:136–48.
Boucher HW, Wilcox M, Talbot GH, et al. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:2169–79.
Ramdeen S, Boucher HW. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Expert Opin Pharmacother. 2015;16:2073–81.
Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis. 2005;41:1407–15.
Rappo U, Gonzalez PL, Puttagunta S, et al. Single-dose dalbavancin and patient satisfaction in an outpatient setting in the treatment of acute bacterial skin and skin structure infections. J Glob Antimicrob Resist. 2019;17:60–5.
Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed 27 June 2019
Acknowledgements
We thank the participants of the study who made the ENHANCE trial possible.
Funding
Sponsorship for this study and the journal’s Rapid Service Fees were funded by Allergan plc (Dublin, Ireland). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing, Editorial, and Other Assistance
Administrative support for article submission was provided by Jennifer Venzie, PhD, and John E. Fincke, PhD, at ICON plc (North Wales, PA), and funded by Allergan plc (Dublin, Ireland). Study support was provided by ICON, a contract research organization.
Authorship
All authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Matthew McCarthy served as a consultant to Allergan and also received research support from Allergan that was paid to Weill Cornell Medicine. Katelyn Keyloun is an employee of Allergan plc. Patrick Gillard is an employee of Allergan plc and holds stock in Allergan plc. Justin Choi has no conflicts to disclose. Nicholas Pickell received Allergan-sponsored support through his employment at Weill Cornell Medicine. Ronald Copp was an employee of ICON plc, the CRO supporting study conduct at the time of study conduct and analysis, and is currently an employee of Array Biostatistics, Wilmington, NC. Thomas Walsh has received grants for experimental and clinical antimicrobial pharmacology and therapeutics to his institution from Allergan, Amplyx, Astellas, Lediant, Medicines Company, Merck, Scynexis, and Tetraphase and has served as consultant to Amplyx, Astellas, Allergan, ContraFect, Gilead, Lediant, Medicines Co., and Merck.
Compliance with Ethics Guidelines
The study was conducted in accordance with the protocol, rules, and regulations of the WCM IRB and standard operating procedures, and sponsor at WCM, and informed consent regulations. Only patients providing informed consent were included in the study. The study followed the hospital’s applicable local regulatory requirements, was governed according to local laws and regulations and was in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10048484.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
McCarthy, M.W., Keyloun, K.R., Gillard, P. et al. Dalbavancin Reduces Hospital Stay and Improves Productivity for Patients with Acute Bacterial Skin and Skin Structure Infections: The ENHANCE Trial. Infect Dis Ther 9, 53–67 (2020). https://doi.org/10.1007/s40121-019-00275-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-019-00275-4