Abstract
A novel Cu–TiO2/chitosan hybrid thin film was successfully prepared without any heat treatment process by sol–gel method on a polycarbonate substrate. Based on the photocatalytic activity and adsorption property of the entire thin film, a simple, reliable, reproducible and inexpensive method was developed for the removal of some heavy metals from aquatic media. The removal process of Pb2+ and Cr6+ was performed by flotation of coated polycarbonate substrate in the bulk of sample for a definite time in absence and presence of light. The effect of influential parameters in removal process, including thin film surface area, removal time, sample volume and pH was investigated and variation in selected ion concentrations was monitored using a graphite furnace-atomic absorption spectrometer. Considering the optimized conditions, effect of interference ions was studied and according relative standard deviation amounts, no evidence of interference was observed. The proposed method showed good reproducibility in a way that the amounts of relative standard deviation percentage values (n = 10) for Pb2+ and Cr6+ were obtained 4.303 and 3.865, respectively. The whole procedure showed to be conveniently applicable and quite easy to manipulate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In recent years, there has been considerable interest in organic/inorganic hybrid membrane composed of polymer matrix and inorganic nanoparticles [1]. The sol–gel method has been proved to be versatile and has been widely used in the preparation of organic–inorganic hybrid materials, non-linear optical materials, and mesomorphous materials [2]. To date, many kinds of inorganic nanoparticles, such as SiO2 [3], Al2O3 [4], Fe3O4 [5], ZnO [6], ZrO2 [7], CdS [8] and TiO2 [9–12] have been introduced into polymer matrix to prepare polymer/inorganic nanoparticles membranes. Among these inorganic nanoparticles, TiO2 has been proved to be excellent fillers because of its good stability, hydrophilicity, resisting and usage as an effective, photostable and reusable catalyst in the photodegradation of heavy metals as well as organic pollutants. It was proved that the standard reduction potential of the metals for the reduction reaction affected on the ability of TiO2 in removal of metals [13]. However, there are still many limitations such as the recollection and reuse of nanometer TiO2. Thin film or the immobilized nanometer TiO2 on different matrices can resolve the above problems [14].
Recently, TiO2/chitosan hybrid thin film is very promising. It is worth mentioning that chitosan is a biodegradable cationic polysaccharide with antimicrobial activity, nontoxic and excellent film-forming ability with good mechanical strength, with its higher permeability and cost-effectiveness caused wide application in the field of sensor [15]. The new double function hybrid thin film resolved the recollection and reuses limitation of nano-TiO2, and was used not only in degradation of dyes, but also in adsorption of heavy metal ions [16–19]. Among heavy metals, lead (II) and chromium (VI) hold distinct position due to their toxicity, long-term and widespread usages [13, 20–23]. Therefore, development of a simple, effective method for the removal of these ions from aquatic media is of great importance.
In this work, Cu–TiO2/chitosan hybrid thin film was successfully prepared without any heat treatment process by sol–gel method on polycarbonate (PC) substrates. Subsequently, photocatalytic activity and adsorption property of the entire thin film were evaluated to the removal of chromium and lead ions from aquatic medium.
Methods
Materials
Chitosan (85 % degree of deacetylation) was purchased from Pelican Biotech and Chemicals Labs. Titanium tetra isopropoxide (TTIP) and Copper(II) Nitrate (both AR analytical grade, Merck Chemical Company) were used as Titanium and Copper sources for the preparation of the Cu–TiO2 nanocomposite. Hydroxy propyl cellulose (HPC) was used as a dispersive agent. Other reagents were analytical grade and purchased locally. Double distilled water was used throughout the study. Furthermore, Pb(NO3)2 and K2Cr2O7, Cd(NO3)2.4H2O, Ni(NO3)2, FeCl3.6H2O and Zn(NO3)2 were all bought from Merck company.
Setup of photocatalytic reactor and removal study
The photocatalytic reduction studies of removal heavy metal were carried out using the photoreactor system, which consisted of a cubic borosilicate glass reactor vessel with an effective volume of 1,000 ml, a cooling water jacket and a 400 W H-P mercury vapor lamp positioned above center of cubic, as a light source. The spectrum of this lamp is shown in Fig. 1. As it shown, large amount of the radiation is located in visible region. The reaction temperature was kept at 25° C by means of cooling water.
To investigate the photocatalytical properties of thin film an amount of 400 ml of a solution containing 50 ppb of Pb2+ or Cr6+ was utilized for removal study while the solution was stirred at 70 % of maximum efficiency of magnetic stirrer. In addition, all experiments were performed in presence and absence of light. Since the allowed levels of both Pb2+ and Cr6+ in drinking water are 5 ppb, all experiments were accomplished based on this level of concentration.
Synthesis of Cu–TiO2/chitosan thin film
The solutions were practically prepared based on the method of Yaghobi et al. [24], and the only difference was the addition of chitosan and Cu2+ to the solution. The four types of Cu–TiO2/chitosan thin films were prepared using the sol–gel method. Solution 1 was a mixture of H2O and H2O2 with volume proportions of 90:200, respectively. Chitosan was dissolved in 3 wt% acetic acid solution at 80 °C and then HPC was added to it (solution 2). Then solution 2 was added drop wise to solution 1 with vigorous stirring (solution 3). Afterwards, 12 ml TTIP was added to solution 3 dropwise with vigorous stirring and following the pH was raised to 7.0. The resulting solution was reflux for 10 h to obtain anatase sol. The four types of Cu–TiO2/chitosan thin films were prepared with some procedure by addition of different agents (refer to Table 1).
The plastic substrates were PC sheets with 1 mm thickness, which are cut into 35 mm × 25 mm pieces. The substrates were washed firstly by water and detergent and then rinsed with double distilled water (DDW). They were further cleaned in 2-propanol using ultrasonication and again rinsed with DDW and finally it was dried. The surface of PC sheets were treated chemically in a solution which is made by dropwise addition of 37.5 ml H2SO4 to 4 g K2Cr2O7 followed by adding 12.5 ml DDW. The substrates were immersed in this solution for 15 min, and then the solution was cooled down to room temperature. Cu–TiO2/chitosan layers were coated on PC substrates using dip coating at the 3 mm/s. For all the samples, a pre-coat of the amorphous TiO2 sol was applied initially on the surface of PC substrates. This layer enhances the adhesion of composite layer and also it acts as a barrier to avoid the photocatalytic degradation of PC.
The preparation of this solution is described in details elsewhere [25]. Briefly, TTIP was dissolved in absolute ethanol with molar ratio TTIP/ethanol/HPC = 1/125/4.5 × 10−3 g g −1sol . Afterward, another mixture of absolute ethanol, HNO3, deionized water, Cu(NO3)2 with molar ratio of ethanol/HNO3/H2O/Nd(NO3)3 = 43/0.2/1/0.002 was added dropwise under vigorous stirring to it. The obtained transparent colloidal suspension was stirred for 45 min and then aged for 48 h to form gel solution. Subsequent layers of samples were deposited by 3 times dip coating. The samples were dried after each dip in a furnace at 110 °C for 15 min.
Characterization
The microstructure of the film samples was observed with a SEM-XL30 scanning electron microscope. The specific surface area of powder was measured by Beijing JWGB Sic. model JW-K. The phase composition of the powders was determined by a SCIFERT-3003 PTS X-ray Diffractometer. The FT-IR analysis was carried out for the samples by Thermo Nicolet NEXUS-870.
The photocatalytic properties of films were recognized by measuring the optical absorption of a 5 ppm methyl orange solution before and after the photodegradation by Varian UV–Vis spectrophotometer. The photocatalytic activity was investigated at the absorption peak (465 nm) before and after the photodegradation.
Results and discussion
The effect of Cu2+ and HPC on the microstructure
The FT-IR spectra of samples are shown in Fig. 2, in wave number range of 4,000–400 cm−1. The band around 3,440, 1,417, 1,421 and 1,418 cm−1 should be assigned to the symmetric vibration of surface hydroxyl group [1, 2, 14, 26]. Hydroxyl band around 2,888 cm−1 is a characteristic of surface TiO2–OH functional group. Furthermore, absorption bands around 1,304 and 1,644 cm−1 were attributed to the amine or amide functional group [26]. The absorption bands around 1,640 and 1,314 cm−1 attributed to the bending vibration of N–H and stretching vibration of C–N, respectively [1, 26]. The band around 620 cm−1 corresponded to the Ti–O–Ti stretching motion [25, 26]. The band change around 1,418 cm−1 is due to hydrogen bond and protonation of the amine groups [2].
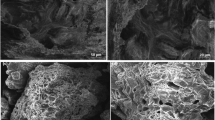
The SEM images are illustrated in Fig. 3. Presence of HPC and Cu2+ is influenced on particle size. Sample 2, which contains HPC, has the wide range of particle distribution with high agglomeration. This could be as a result of not using heat treatment process. Cu2+ as a doping element was added to nanocomposite. In presence of Cu2+, nanocomposite particle size decreases and distribution range lowered and coating accomplished monotonously. To sum up, among these samples, samples 3 and 4 show improved uniformity. However, sample 3 was selected for removal study because of its loose structure.
The effect of Cu2+ and HPC on the anatase phase
XRD pattern of sample 3 is shown in Fig. 4. The XRD measurements revealed that the sample possessed an anatase structure, and there was no evidence of rutile and mixed phases. Although the amount of anatase phase is not significant, it is important that this phase was formed without any heat treatment, which is a common process after sol–gel method.
The self-cleaning ability of nanocomposite was investigated, as well. According to Fig. 5, the methyl orange absorption decreased during contact time with nanocomposite thin film.
Removal study using nanocomposite thin film
Parameters affecting removal process including thin film surface area, removal time, sample volume and pH were studied. Effect of interference ions along with reproducibility of method was investigated as well. Variation in selected ion concentrations was monitored using a graphite furnace-atomic absorption spectrometer.
Study on sorbent surface area and time of removal in presence and absence of light
Removal of lead ion was investigated using various surface areas including 131, 218, 306, 437 cm2 of prepared nanocomposite thin film during 4 h. To study removal time, sampling was accomplished each 30 min while sample was exposed to light. Variation in lead concentration was monitored at room temperature. The entire test was also carried out at the same condition in absence of light. According to Fig. 6a, among these surface areas, 131 cm2 caused minimum removal recovery. From the economic point of view, using less amount of sorbent was used to decrease the removal time. Therefore, sorbent surface area of 218 cm2 was selected.
As Fig. 6a shows removal time effect, there is no significant change after 60 min. So, this time chose as the optimum removal time. According to Fig. 6b, the investigation on removal recovery in absence of light resulted in the same optimum condition.
The effect of removal time was also studied for Cr(VI) using thin film with 218 cm2 surface area. As shown in Fig. 7a, there is a considerable increase in removal efficiency up to 90 min for Cr3+ and slight changes observed after this time while sample exposed to the light. As it is illustrated in Fig. 7b, the removal efficiency for this ion in absence of light increased up to 150 min. After this time, the extraction efficiency was not increased significantly. Beyond longer equilibrium time, comparing results shows a more rapid removal rate for Cr3+ in presence of light. It was proved that adsorption of Cr(VI) on TiO2 in darkness is low and, therefore, the recovery of elimination process decreases in absence of light. Moreover, the amount of Cr(VI) adsorption raises in some extent with increasing irradiation time [27]. Since similar behavior was observed for Pb2+, it might be same mechanism happened.
Sample size effect
To study the effect of sample size, different volumes of solutions including 200, 300, 400, 500, 700, 800 ml was used. All these solutions were containing 50 ppb of both Cr3+ and Pb2+ ions and each solution exposed to 218 cm2 of sorbent while it stirred. As the results shown in Fig. 8a and b, the volumes of more than 400 ml resulted in significant decrease in removal efficiency. It seems that saturation of sorbent could lead to this decline. So the volume of 400 ml was selected for further studies.
pH effect
Since lead and chromium ions are precipitated and polymerized in alkaline media as Pb(OH)2, Cr(OH)3, Cr2(OH) 4+2 , Cr3(OH) 5+4 , the study on the effect of this parameter is accomplished under neutral and acidic condition in presence and absence of light. Therefore, the amount of 400 ml solutions containing 50 ppb of Pb2+ and Cr3+ with different pH in the range of 1–7 was prepared and then each solution was exposed to the sorbent with the surface area of 218 cm2 for 4 h. As the results shown (Fig. 9a, b), an improvement in removal recoveries is observed in pH 2 for Pb2+ and Cr3+, when the sample exposed to the light. Decrease in the recoveries after and before the entire pH could be attributed to the solvation, hydrolysis, polymerization of these ions and occupation of active sites of chitosan by H+, respectively. In addition, it was proved that photocatalytic reduction of Cr(VI) in presence of TiO2 was very sensitive to pH and this process is most efficient less than pH 3. At pH 5, separation of the catalyst and water occurred as Cr(III) ion formed a stable precipitate on the catalyst. The proposed reaction for the process was shown according reaction (1):
Prairie et al. [27] revealed that thermodynamic driving force for Cr(VI) reduction on TiO2 in basic aqueous solution was less than in acidic media. In addition, comparing the results in Fig. 9 a and b shows significant removing recoveries in presence of light for both ions.
Method performance
Investigation of interference ions
Since adsorption is a competitive phenomenon, the presence of other ions could affect on removal recovery. It is worth mentioning that interference ion is considered the one, which could affect on removal signal more than 5 %.
Firstly, the effect of chromium ion on adsorption of Pb2+ in both presence and absence of light was investigated. For this study, an amount of 400 ml solution containing 50 ppb of each ion was used and sampling under optimized condition was accomplished. As shown in Table 2, the removal process using nanocomposite thin film in presence of chromium ion was performed more significantly, while the sample was exposed to the light. Furthermore, there is no evidence of interference according to RSD amounts in both conditions.
Subsequently, same study was done in solutions containing 50 ppb of Pb2+ or Cr3+ along with the amounts of 5 ppm of Cu2+, Cd2+, Zn2+, Fe2+ and Ni2+ ions in 400 ml solution. The removal process was evaluated both under optimized condition for lead and chromium. As Tables 3 and 4 illustrated, the studied ions caused just a slight decrease in removal of both analytes in concentration level of 100 times more than their amounts. This decline could be attributed to occupation of chitosan active sites by the investigated ions.
The results proved steady rising of removal recovery in presence of light. In addition, the dramatic potential of nanocomposite thin film for removing lead ion in comparison with chromium ions is sensible.
Reproducibility of method
Considering the optimized conditions, reproducibility of proposed method was assessed for each ion using 10 replicates. The amounts of RSD% were 4.303 and 3.865 for Pb2+ and Cr6+, respectively.
Conclusions
The primary aim of this study was to develop a simple, reliable, reproducible and inexpensive method for the removal of heavy metals from aquatic environmental samples using photocatalytic reduction. To achieve this purpose, adsorption of lead and chromium ions on the surface of newly synthesized photocatalyst thin film, Cu–TiO2/chitosan nanocomposite, was investigated. The Cu–TiO2/chitosan nanocomposite film was prepared by sol–gel method without any heat treatment process. Presence of Cu2+ as a dopant ion improves photocatalytic activity and could also increase adsorption property. The synthesized catalyst was coated on the surface of polycarbonate substrate which provides higher specific surface area and could float readily in the bulk of sample. In comparison with powdered catalysts, using thin film causes readily removing of catalyst from solution without any residue and could decline significantly the cost of water purification process as well. Influential parameters in removal recovery of selected heavy metals based on photocatalytic process were optimized and under this condition, the effects of other ions on removal recoveries were investigated and no evidence of interference was found. Furthermore, the results proved increase in removal recoveries in presence of light along with appropriate reproducibility. Considering feasibility of established method, selective removal of heavy metals could be possible using different doping agents to the entire catalyst.
Abbreviations
- TTIP:
-
Titanium tetra isopropoxide
- HPC:
-
Hydroxy propyl cellulose
- H–P:
-
High pressure
- DDW:
-
Double distilled water
- PC:
-
Polycarbonate
- SEM:
-
Scanning electron microscope
- FT-IR:
-
Fourier transform infrared
References
Yang, D., Li, J., Jiong, Zh, Lu, L., Chen, X.: Chitosan/TiO2 nanocomposite pervaporation membranes for ethanol dehydration. Chem. Eng. Sci. 64, 3130–3137 (2009)
Tao, Y., PanYan, J.S., Tang, B., Zhu, L.: Tensile strength optimization and characterization of chitosan/TiO2 hybrid film. Mater. Sci. Eng. B 138, 84–89 (2007)
Khayet, M., Villaluenga, J.P.G., Valentin, J.L., López-Manchado, M.A., Mengual, J.A., Seoane, B.: Filled poly (2,6-dimethyl-1,4-phenylene oxide) dense membranes by silica and silane modified silica nanoparticles: characterization and application in pervaporation. Polymer 47, 114–122 (2005)
Shen, J.Y., Zhu, W.T., Chen, L.Q., Qiu, X.P.: A nanocomposite proton exchange membrane based on PVDF, poly(2-acrylamido-2-methyl propylene sulfonic acid), and nano-Al2O3 for direct methanol fuel cells. J. Power Sources 159, 894–899 (2006)
Han, P., Yahui, H., Yang, W., Linlin, L.: Preparation of polysulfone-Fe3O4 composite ultrafiltration membrane and its magnetic field. J. Membr. Sci. 284, 9–16 (2006)
Huang, H.G., Chen, J.H., Zou, L.C.: Preparation and characterization of ZnO–PANI composite film. J. Rare Met. 27, 91–94 (2003)
Bottino, A., Capannelli, G., Comite, A.: Preparation and characterization of novel porous PVDF-ZrO2 composite membranes. Desalination 146, 35–40 (2002)
Trigo, C.E.L., Porto, A.O., de Lima, G.M.: Preparation and characterization of ZnO–PANI composite film. Eur. Polym. J40, 2465–2469 (2004)
Vona, M.L.D., Ahmed, Z., Bellitto, S., Lenci, A., Traversa, E., Licencia, S.: SPEEK-TiO2 nanocomposite hybrid proton conductive membranes via in situ mixed sol–gel process. J. Membr. Sci. 269, 156–161 (2007)
Wu, Z., Suna, G., Jin, W., Hou, H., Wang, S., Xin, Q.: Nafion and nano-size TiO2–SO42− solid superacid composite membrane for direct methanol fuel cell. J. Membr. Sci. 313, 336–343 (2008)
Yang, C.C.: Synthesis and characterization of the cross-linked PVA/TiO2 composite polymer membrane for alkaline DMFC. J. Membr. Sci. 288, 51–60 (2007)
Zhou, H., Chen, Y., Fan, H., Shi, H., Luo, Z., Shi, B.: Water vapor permeability of the polyurethane/TiO2 nanohybrid membrane with temperature sensitivity. J. Appl. Polym. Sci. 109, 3002–3007 (2008)
Kabra, K., Chaudhary, R., Sawhney, R.L.: Treatment of hazardous organic and inorganic compounds though aqueous-phase photocatalysis: a review. Ind. Eng. Chem. Res. 43, 7683–7696 (2004)
Li, Q., Su, H., Tan, T.: Synthesis of ion-imprinted chitosan–TiO2 adsorbent and its multifunctional performances. Biochem. Eng. J. 38, 212–218 (2008)
Vargas, M., Albors, A., Chiralt, A., González-Martinez, C.: Characterization of chitosan oleic acid composite films. Food Hydrocoll. 23, 536–547 (2009)
Zhang, X., Zhao, X., Su, H.: Degradation characteristic of TiO2-Chitosan adsorbent on Rhodamine B and purification of industrial wastewater. J. Chem. Eng. 28, 1241–1246 (2011)
Liu, Z., Bai, H., Delai Sun, D.: Facile fabrication of porous chitosan/TiO2/Fe3O4 microspheres with multifunction for water purifications. New J. Chem. 35, 137–140 (2011)
Buraidah, M.H., Teo, L.P., Yusuf, S.N.F, Noor, M.M., Kufian, M.Z., Careem, M.A., Majid, S.R., Taha, R.M., Arof, A.K.: TiO2/chitosan-NH4I(+I2)-BMII-based dye-sensitized solar cells with anthocyanin dyes extracted from black rice and red cabbage. Int. J. Photoenergy 2011, 1–11 (2011)
Zubieta, C.E., Messina, P.V., Luengo, C., Dennehy, M., Pieroni, O., Schulz, P.C.: Reactive dyes remotion by porous TiO2-chitosan materials. J. Hazard Mater. 152, 765–777 (2008)
Ahmad, A., Ghufran, R., Faizal, W.M.: Cd(II), Pb(II) and Zn(II) removal from contaminated water by biosorption using activated sludge biomass. Clean 38(2), 153–158 (2010)
Tao, Y., Ye, L., Pan, J., Wang, Y., Tang, B.: Removal of Pb(II) from aqueous solution on chitosan/TiO2 hybrid film. J. Hazard Mater. 161, 718–722 (2009)
Colón, G., Hidalgo, M.C., Navio, J.A.: Effect of ZrO2 incorporation and calcinations temperature on the photocatalytic activity of commercial TiO2 for salicylic acid and Cr(VI) photodegradation. Appl. Catal. A Gen. 231, 185–199 (2002)
Selli, E., Giorgi, A., Bidoglio, G.: Humic acid-sensitized photoreduction of Cr(VI) on ZnO particles. Environ. Sci. Technol. 30, 598–604 (1996)
Yaghoubi, H., Taghavinia, N., Keshavarz Alamdari, E.: Self cleaning TiO2 coating on polycarbonate: surface treatment, photocatalytic and nanomechanical properties. Surf. Coat. Technol. 204, 1562–1568 (2010)
Aberoomand Azar, P., Moradidehaghi, Sh, Samadi, S., Saber Tehrani, M., Givianrad, M.H.: Effect of CMC and HPC mixture on the photocatalytic activity of Nd-TiO2/SiO2 film under visible light irradiation. Turk. J. Chem. 35, 37–44 (2011)
Gomez-Serrano, V., Macia, A., Spinosa-Mansilla, A., Valenzuela-Calahorro, C.: Adsorption of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res. 32, 1–4 (1998)
Prairie, M.R., Evans, L.R., Martinez, S.L.: Destruction of organics and removal of heavy metals in water via TiO2 photocatalysis. In: Eckenfelder, W.W., Bowers, A.R., Roth, J.A. (eds.) Chemical Oxidation: Technology for the Nineties. Second International Symposium, pp. 428–441. JA Technomic Publishing Company, Lancaster, PA (1994)
Acknowledgments
The author is grateful to the experts of Laboratory of Shar-e-Rey Branch, Islamic Azad University for valuable technical assistance.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Samadi, S., Khalilian, F. & Tabatabaee, A. Synthesis, characterization and application of Cu–TiO2/chitosan nanocomposite thin film for the removal of some heavy metals from aquatic media. J Nanostruct Chem 4, 84 (2014). https://doi.org/10.1007/s40097-014-0084-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40097-014-0084-3