Abstract
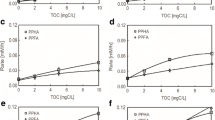
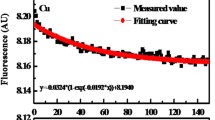
Redox properties of humic substances (HS) control important biogeochemical processes. Thus, accurate estimation of redox properties of HS is essential. However, there is no general consensus regarding the best available measurement method of HS redox properties. In this study, we compared several common HS redox property measurement methods using anthraquinone-2,6-disulfonate (AQDS) as model compound, and standard Elliot soil humic acid (1S102H, ESHA), reference Pahokee peat (1R103H, PPHA), and Suwannee River natural organic matter (1R101N, SRNOM) as representative HS. We found that the H2/Pd reduction method followed by incubation with ferric citrate (FeCit) reagent was incomplete, and the H2/Pd reduction method followed by incubation with potassium ferricyanide (K3Fe(CN)6) was insensitive. Stannous chloride (SnCl2) reduction followed by titration of excess stannous (Sn2+) by potassium dichromate (K2Cr2O7) was found to be most accurate. These findings will help in future investigations on detailed characterizations of functional groups of HS responsible for oxidation/reduction reactions.










Similar content being viewed by others
References
Adhikari D, Poulson SR, Sumalia S, Dynes JJ, McBeth JM, Yang Y (2016) Asynchronous reductive release of iron and organic carbon from hematite-humic acid complexes. Chem Geol 430:13–20
Aeschbacher M, Sander M, Schwarzenbach RP (2010) Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol 44:87–93
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell KM (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Chen J, Gu B, Royer RA, Burgos WD (2003) The roles of natural organic matter in chemical and microbial reduction of ferric iron. Sci Total Environ 307:167–178
de Melo BAG, Motta FL, Santana MHA (2016) Humic acids: structural properties, and multiple functionalities for novel technological developments. Mater Sci Eng C 62:967–974
Diaz AN (1990) Absorption and emission spectroscopy and photochemistry of 1,10-anthraquinone derivatives: a review. J Photochem Photobiol A 53:141–167
Dunnivant FM, Schwarzenbach RP, Macalady DL (1992) Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol 26:2133–2141
Gautrot JE, Hodge P, Cupertino D, Helliwell M (2007) 2, 6-Diaryl-9,10-anthraquinones as models for electron-accepting polymers. New J Chem 31:1585–1593
Hernandez-Monotoya V, Alvarez LH, Montes-Moran MA, Cervantes FJ (2012) Reduction of quinone and non-quinone redox functional groups in different humic acid samples by Geobacter sulfurreducens. Geoderma 183–184:25–31
Irving HMNH (1978) Recommendations on the usage of the terms ‘Equivalent’ and ‘Normal’. Pure and Appl Chem 50:325–338
Jiang J, Bauer I, Paul A, Kappler A (2009) Arsenic redox change by microbially and chemically formed semiquinone radicals and hydroquinones in a humic substance model quinone. Environ Sci Technol 43:3639–3645
Kappler A, Haderlein SB (2003) Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol 37:2714–2719
Kappler A, Benz M, Schink B, Brune A (2004) Electron shuttling via humic acids in microbial iron(II) reduction in freshwater sediment. FEMS Microbiol Ecol 47:85–92
Klüpfel L, Pipenbrock A, Kappler A, Sander M (2014) Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat Geosci 7:195–2000
Lovley DR, Blunt-Harris EL (1999) Role of humic-bound iron as an electron transfer agent in dissimilatory Fe(III) reduction. Appl Environ Microbiol 65:4252–4254
Macalady DL, Walton-Day K (2011) Redox chemistry and natural organic matter (NOM): geochemists’ dream, analytical chemists’ nightmare. In: Tratnyek PG, Grundl TJ, Haderlein SB (eds). American Chemical Society, Washington, pp 85–111
Matthiessen A (1995) Determining the redox capacity of humic substances as a function of pH. Vom Wasser 84:229–233
Nevin KP, Lovley DR (2000) Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Environ Sci Technol 34:2472–2478
Peretyazhko T, Sposito G (2006) Reducing capacity of terrestrial humic acids. Geoderma 137:140–146
Peters RH, Sumner HH (1953) Spectra of anthraquinone derivatives. J Chem Soc 2102–2110. doi:10.1039/JR9530002101
Pipenbrock A, Schröder C, Kappler A (2014) Elelctron transfer from humic substances to biogenic and abiogenic Fe(III) oxyhydroxy minerals. Environ Sci Technol 48:1656–1664
Rakshit S, Uchimiya M, Sposito G (2009) Iron(III) bioreduction in soil in the presence of added humic substances. Soil Sci Soc Am J 73:66–71
Ratasuk N, Nanny MA (2007) Characterization and quantification of reversible redox sites in humic substances. Environ Sci Technol 41:7844–7850
Schnitzer M, Riffaldi R (1972) The determination of quinone groups in humic substances. Soil Sci Soc Am Proc 36:772–777
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol 32:2984–2989
Sposito G (2011) Electron shuttling by natural organic matter: twenty years after. In: Tratnyek PG, Grundl TJ, Haderlein SB (eds) American Chemical Society, Washington, pp 113–127
Stookey LL (1970) Ferrozine: a new spectrometric reagent for iron. Anal Chem 42:779–781
Vasilyevskaya NA, Glebko LI, Maximov OB (1971) Determination of quinoid groups in humic acids. Pochvovdenie 4:63–67 (in Russian)
Visser SA (1964) Oxidation-reduction potentials and capillary activities of humic acids. Nature 204:581
Weber KA, Achenbatch LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–763
Yang Z, Du M, Jiang J (2016) Reducing capacities and redox potentials of humic substances extracted from sewage sludge. Chemosphere 144:902–908
Acknowledgements
This research was supported by the Evans-Allen Grant from USDA and NSF Grant DEB-0543558. The authors express gratitude to Professor Garrison Sposito at the University of California, Berkeley, for providing laboratory facilities to conduct experimental work for completing this manuscript. The authors also thank Mr. Andrew Yang for his help in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: R. Datta.
Rights and permissions
About this article
Cite this article
Rakshit, S., Sarkar, D. Assessing redox properties of standard humic substances. Int. J. Environ. Sci. Technol. 14, 1497–1504 (2017). https://doi.org/10.1007/s13762-017-1263-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1263-9