Abstract
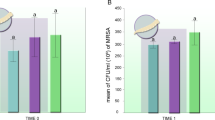
Maggot debridement therapy (MDT) consists on the intentional and controlled application of sterilized larvae of the order Diptera on necrotic skin lesions with the purpose of cleaning necrotic tissue and removing pathogenic bacteria. During MDT, a marked antimicrobial activity has been reported in literature specially associated with antibacterial substances from Lucilia sericata (Meigen); however, regarding Cochliomyia macellaria (Fabricius), little is known. This study aimed to evaluate in vitro inhibition of bacterial growth of Pseudomonas aeruginosa and Staphylococcus aureus in contact with excretions and secretions (ES) from C. macellaria larvae. Larval ES were extracted in sterile distilled water and divided in three groups: ES, containing 400 μL of autoclaved ES; ES+BAC, containing 400 μL of autoclaved ES+0.5-μL bacterial inoculum; and CONT-BAC, containing 400 μL of sterile distilled water +0.5 μL of bacterial inoculum. Aliquots of each experimental group were plated by spreading onto Petri dishes. Seedings were made at 0, 1, 2, 4, and 12 h after the extraction of ES. In ES+BAC groups, inhibition of S. aureus was verified between times 1 and 2 h and P. aeruginosa was inhibited between 0 and 4 h. There was no growth observed in any ES group. In the CONT-BAC groups, the number of colonies from time 4 h became countless for S. aureus and decreased for P. aeruginosa. As reported in the literature, we note here that ES have excellent bactericidal activity for both gram-positive and gram-negative bacteria, and this study shows for the first time the action of the bactericidal activity of exosecretions of C. macellaria against S. aureus and P. aeruginosa.


Similar content being viewed by others
References
Andersen A, Sandvang D, Schnorr K, et al. (2010) A novel approach to the antimicrobial activity of maggot debridement therapy. J Antimicrob Chemother 65:1646–1654
Arora S, Sing LC, Baptista C (2010) Antibacterial activity of Lucilia cuprina maggot extracts and its extraction techniques. Int J Integr Biol 9(1):43–48
Arora S, Baptista C, Lim CS (2011) Maggot metabolites and their combinatory effects with antibiotic on Staphylococcus aureus. Ann Clin Microbiol Antimicrob 10:1–8
Baer WS (1931) The treatment of chronic osteomyelitis with the maggot (larva of the blowfly). J Bone Joint Surg Am 13:438–475
Barnes KM, Gennard DE, Dixon RA (2010) An assessment of the antibacterial activity in larval excretion/secretion of four species of insects recorded in association with corpses, using Lucilia sericata Meigen as the marker species. Bull Entomol Res 100:635–640
Bexfield A, Nigam Y, Thomas S, Ratcliffe NA (2004) Detection and partial characterization of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microbes Infect 6:1297–1304
Bexfield A, Bond AE, Morgan C, et al. (2009) Amino acid derivatives from Lucilia sericata excretions⁄secretions may contribute to the beneficial effects of maggot therapy via increased angiogenesis. Brit J Dermatol 162:554–562
Bjarnsholt T, Kirketerp-Moller K, Jensen PO, et al. (2008) Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 16:2–10
Brooks GF, Butel JS, Ornston LN (2000) Microbiologia Médica. Artmed, Porto Alegre
Brown A, Horobin A, Blount DG, Hill PJ, English J, et al. (2012) Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med Vet Entomol 26:432–439
Cazander G, Van Veen K, Bernards A, Jukema G (2009) Do maggots have an influence on the bacterial growth? A study on the susceptibility of strains of five different bacterial species to maggots of Lucilia sericata. J Tissue Viability 18:80–87
Cazander G, van de Veerdonk MC, Vandenbroucke-Grauls CM (2010) Maggot excretions inhibit biofilm formation on biomaterials. Clin Orthop Relat Res 468:2789–2796
Chambers L, Woodrow S, et al. (2003) Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol 148:14–23
Erdmann GR, Khalil SK (1986) Isolation and identification of two antibacterial agents produced by a strain of Proteus mirabilis isolated from larvae of the screwworm (Cochliomyia hominivorax) (Diptera: Calliphoridae). J Med Entomol 23:208–211
Erdmann E, Werdan K, Krawietz W, Schmitz W, Scholz H (1984) Vanadate and its significance in biochemistry and pharmacology. Biochem Pharmac 33:945–950
Fleischmann W, Grassberger M, Sherman R (2004) A handbook of maggots-assisted wound healing, New York
Greenberg B (1968) Model for destruction of bacteria in the midgut of blowfly maggots. J Med Entomol 5:31–38
Grella MD, Thyssen PJ (2011) Chave taxonômica interativa para espécies de dípteros califorídeos (Infraordem: Muscomorpha) do Brasil. http://keys.lucidcentral.org/keys/v3/calliphoridae_brazil. Accessed 21 October 2014
Guimarães JH, Papavero N, Prado AP (1983) As miíases na região neotropical. Rev Bras Zool 1:239–416
Harris LG, Nigam Y, Sawyer J, Mack D, Pritchard DI (2013) Lucilia sericata chymotrypsin disrupts protein adhesin-mediated staphylococcal biofilm formation. Appl Environ Microbiol 79:1393–1395
Jaklic D, Lapanje A, Zupancic K, Smrke D, Gunde-Cimerman N (2008) Selective antimicrobial activity of maggots against pathogenic bacteria. J Med Microbiol 57:617–625
Jiang KC, Sun XJ, Wang W, Liu L, Cai Y, Chen YC, Luo N, Yu JH, Cai DY, Wang AP (2012) Excretions/secretions from bacteria-pretreated maggot are more effective against Pseudomonas aeruginosa biofilms. PLoS One 11:1–4
Kawabata T, Mitsui H, Yokota K, Ishino K, Oguma K, et al. (2010) Induction of antibacterial activity in larvae of the blowfly Lucilia sericata by an infected environment. Med Vet Entomol 24:375–381
Kerridge A, Lappin-Scott H, Stevens JR (2005) Antibacterial properties of larval secretions of the blowfly, Lucilia sericata. Med Vet Entomol 19:333–337
Kruglikova AA, Chernysh SI (2011) Antimicrobial compounds from the excretions of surgical maggots, Lucilia sericata (Meigen) (Diptera, Calliphoridae). Entomol Rev 91:813–819
Masiero FS, Thyssen PJ (2016) Evaluation of conventional therapeutic methods versus maggot therapy in the evolution of healing of tegumental injuries in Wistar rats with and without diabetes mellitus. Parasitol Res. doi:10.1007/s00436-016-4991-8
Masiero FS, Nassu MP, Soares MP, Thyssen PJ (2015) Histological patterns in healing chronic wounds using Cochliomyia macellaria (Diptera: Calliphoridae) larvae and other therapeutic measures. Parasitol Res 111:2865–2872
Moretti TC, Thyssen PJ, Solis DR (2009) Breeding of the scuttle fly Megaselia scalaris in a fish carcass and implications for the use in forensic entomology (Diptera: Phoridae). Entomol Gen 31:349–353
Mumcuoglu KY, Miller J, Mumcuoglu M, Friger M, Tarshis M (2001) Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae). J Med Entomol 38:161–166
Nassu MP, Thyssen PJ (2015) Evaluation of larval density Cochliomyia macellaria F. (Diptera: Calliphoridae) for therapeutic use in the recovery of tegumentar injuries. Parasitol Res 114:3255–3260
Okuma K, Iwakawa K, et al. (2002) Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol 40(11):4289–4294
Pavillard ER, Wright EA (1957) An antibiotic from maggots. Nature 180:916–917
Prete PE (1997) Growth effects of Phaenicia sericata larval extracts on fibroblasts: mechanism for wound healing by maggot therapy. Life Sci 60:505–510
Ratcliffe NA, Vieira CS, Mendonça PM, Caetano RL, Queiroz MMC, Garcia ES, Mello CB, Azambuja P (2015) Detection and preliminary physico-chemical properties of antimicrobial components in the native excretions/secretions of three species of Chrysomya (Diptera, Calliphoridae) in Brazil. Acta Trop 147:6–11
Robinson W, Norwood VH (1934) Destruction of pyogenic bacteria in the alimentary tract of surgical maggots implanted in infected wounds. J Lab Clin Med 19:581–586
Sherman R (2009) Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol 3:336–344
Simmons S (1935) A bactericidal principle in excretions of surgical maggots which destroys important etiological agents of pyogenic infections. J Bacteriol 30:253–267
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209
Thomas S, Andrews AM, Hay NP, Bourgoise S (1999) The anti-microbial activity of maggot secretions: results of a preliminary study. J Tissue Viability 9:127–132
Van Der Plas M, Jukema G, Wai S, Dogterom-Hallering H, Lagendijk E (2008) Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother 61:117–122
Acknowledgments
Financial support grant: 2012/06033-5, São Paulo Research Foundation (FAPESP) and Coordination for the Improvement of Education Personnel (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Simão Dias Vasconcelos – UFRPE
Rights and permissions
About this article
Cite this article
Masiero, F.S., Aquino, M.F.K., Nassu, M.P. et al. First Record of Larval Secretions of Cochliomyia macellaria (Fabricius, 1775) (Diptera: Calliphoridae) Inhibiting the Growth of Staphylococcus aureus and Pseudomonas aeruginosa . Neotrop Entomol 46, 125–129 (2017). https://doi.org/10.1007/s13744-016-0444-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0444-4