Abstract
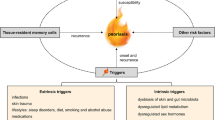
Multifactorial heredity, involving interaction between genetic and environmental factors, is the model hypothesized for psoriasis causation. Measures of heritability indicate that from 50 % to 90 % of the psoriatic phenotype is connected with genetic factors. The remaining variability is due to environmental factors. Candidate risk factors can be initially identified by analyzing variations in population incidence or prevalence. A trend toward increasing incidence of psoriasis in recent decades has been documented. Analytic epidemiologic studies offer a better insight into risk factors allowing to obtain quantitative estimates of the contribution of a factor of interest to the disease and to control for potential confounders. Smoking habits, alcohol consumption, diet, obesity, physical inactivity, infection, drugs, and stressful life events have been considered as candidate risk factors. Convincing proofs exist for only a few of them, namely smoking and obesity. Knowledge of potentially avoidable risk factors may offer clues to plan preventive measures.
Similar content being viewed by others
Why Study Risk Factors?
In epidemiology, a “risk factor” (sometimes referred to also as a “determinant”) is a variable associated with an increased risk or probability of developing a disease. Similarly, a “protective factor” acts by reducing the disease risk. The term "risk factor" was first coined by William B. Kannel, former Framingham Heart Study Director, in 1961 [1]. The concept implies a complex model of disease causation where the occurrence of a disease depends on interplay between multiple associated variables that could be measured by the extent to which they affect the probability of disease occurrence. Hints on the role of environmental risk factors can be provided by simple descriptive epidemiology studies (e.g., ecological correlations); however, risk confirmation and quantitative estimates could only be obtained by analytic epidemiology studies, i.e., cohort and case-control studies. Cohort studies are studies where groups are defined according to the exposure status (e.g., smokers vs. nonsmokers) and are followed up with subsequent events (e.g., psoriasis) being recorded and compared. On the contrary, case-control studies are studies where groups are defined according to their experience of an outcome of interest (e.g., newly diagnosed psoriasis patients and their neighbor controls) and prior exposures are ascertained retrospectively and compared. The association of an exposure with a given event is usually expressed in terms of a “relative risk,” “hazard ratio” (a measure of risk in survival analyses), or “odds ratio” (an estimate of the relative risk obtained from case-control studies). The relative risk is a measure of the size of an association in relative terms. It refers to the ratio of the incidence of the outcome among exposed individuals to that among nonexposed. Values greater than 1 represent an increase in risk associated with the exposure, whereas values less than 1 represent a reduction in risk. A relative risk of two, for example, tells us that the event under study occurs two times more often in the exposed people than in nonexposed. When performing epidemiologic studies to evaluate one or more determinants for a specific disease, the other determinants may act as so-called “confounding factors” (or “confounders”) and need to be controlled for, e.g., by stratification or multivariate analysis. Potential confounders vary with what outcome is studied, but some general confounders, such as age and gender, are common to most epidemiological associations and are the determinants which should be regularly controlled for in epidemiological studies.
Statistical analysis alone is usually not sufficient to establish disease determinants. The Bradford Hill criteria have been proposed as a group of minimal conditions necessary to provide adequate evidence of a causal relationship. They include the strength of the association (e.g., size of the relative risk), consistency, specificity, temporal relationship, biological gradient (e.g., dose-response relationship), biological plausibility, coherence, and further experimental evidence [2].
Multifactorial heredity, i.e., interaction between genetic predisposition and environmental factors, is the model hypothesized for the causation of several chronic inflammatory disorders, including psoriasis. According to the model, the susceptibility to psoriatic lesions depends on both genetic and extra-genetic predisposing factors and is shared by the whole skin. Disease expression, in terms of visible lesions, is modulated by extra-genetic precipitating or initiating factors. Knowledge of avoidable environmental factors may offer clues to plan preventive measures. In addition, environmental influences should be carefully considered in genetic studies, because they can modify the association with genetic markers.
Hints on Risk Factors from Descriptive Epidemiology
Candidate risk factors can be first identified by analyzing variations in population incidence or prevalence measures. A special case of these analyses are ecological correlations, which explore the relationship between two variables that are group means rather than measures that describe individuals. For example, one might study the correlation between average population consumption of fish and incidence or prevalence of psoriasis in different geographic areas or ethnic groups. Unfortunately, the limitation of data concerning the incidence or prevalence of psoriasis hampers most of these exploratory analyses [3].
Ethnic and Geographic Variations
Geographic and ethnic variations are observed for psoriasis. Mongoloid races in the Far East of Asia have remarkably low prevalence rates [4••]. Lower prevalence rates have been also documented in African Americans compared with Caucasians in United States [5•]. Duffy et al. [6], analyzing cumulative incidence in 3,808 twin pairs, documented significantly higher prevalence rates in southern states of Australia with respect to northern areas. Geographical variations were also described by Braathen et al. [7] with the northern regions of Troms and Finmark showing higher rates and Hedmark and Oppland regions in the south of the country showing lower rates.
Sex, Age, and Time Variations
Most prevalence studies suggest that psoriasis tends to be slightly more prevalent among men compared with women. The few studies available that provide age-specific incidence rates of psoriasis suggest that incidence increases more or less steadily with age up to the seventh decade of life. If psoriasis appeared throughout life, then both point prevalence and lifetime prevalence would increase with age. However, prevalence estimates in several studies do not increase with age and even decreases [6–8], suggesting higher mortality rates in older patients with psoriasis compared with the general population. Age variations in morbidity are expected for many diseases and their biological basis are generally complex [9]. A small proportion of common chronic disorders may be due to simple Mendelian mechanisms, and they typically manifest at an earlier age compared with the usual type. Age-at-onset distribution may be employed to test hypotheses about etiologic factors. Evidence of bimodality suggests etiologic heterogeneity and lognormal distribution (Sartwell’s model) [9, 10] a single-gene disorder (age-at-onset for genetic disorders is the equivalent of the incubation period in infectious diseases). In the past, much emphasis has been placed on age-at-onset in discriminating between different types of psoriasis. It has been reported that age-at-onset in large series of psoriatic patients has a bimodal distribution [11]. This has been taken as evidence for etiologic heterogeneity and type I and type II psoriasis have been proposed. Patients with onset of psoriasis before age 40 years (type I psoriasis) are much more likely than patients with onset after age 40 years (type II psoriasis) to have affected first-degree relatives, to express susceptibility alleles at the HLA locus, and to experience severe and recurrent disease. This is an interesting concept. However, aside from study design issues, such as case definition and selection (e.g., the selection of juvenile cases may conditioned by their family history more frequently than cases in the older age groups), a fundamental problem in these analyses arises from the fact that the numerical distribution by age of cases of psoriasis is a function of the age-specific disease rate and the age distribution of the population. That is, variations in numerator data, i.e., the number of people experiencing onset at different ages, may simply reflect the age distribution of the population of origin. In my opinion, age-specific rates of appearance of psoriasis calculated on representative samples of the general population would be more convincing. A trend of increasing incidence during a 30-year period, between 1970 and 2000, has been recently documented for both adult-onset psoriasis and psoriatic arthritis in studies from the Olmsted County, Minnesota [12••, 13••]. This increasing trend may reflect a variety of factors, including a true change in risk factors (e.g., obesity) or changes in the diagnosing patterns. Incidence in males displayed a slightly bimodal pattern but this was not significant. Among female subjects, a prominent increase in incidence in the sixth decade of life that corresponds to the postmenopausal period was documented.
Familial Aggregation
A history of psoriasis in first-degree relatives is given by 20-30 % of patients with psoriasis. In a study, the prevalence of psoriasis increased with the number of first-degree relatives affected from 3 % with no relative affected, to approximately 40 % with two relatives affected [8].
Risk Estimates from Analytic Epidemiology
Genetic Factors
The role of genetic factors is well established. Their review is outside the scope of this chapter. Heritability quantifies the overall role of genetic factors when a multifactorial model of inheritance is postulated, i.e., phenotype is a linear function of independent genetic and environmental factors. When the phenotype is discrete (i.e., affected versus non-affected), the statistical model assumes that an underlying continuous trait (termed “liability”) dictates risk of disease. Heritability is a ratio and its measures lie between 0 and 1.0. Measures of the heritability of psoriasis have been provided based on population data and the analysis of concordance of twins. The estimates ranged from 0.5 to 0.9 [6, 8, 14], indicating that from 50 % to 90 % of the psoriatic phenotype is connected with genetic factors. The highest estimates come from twin studies. Twins are uniquely matched for genetic factors, and they provide an upper estimate of heritability. For comparison, heritability estimates of rheumatoid arthritis range from 0.5 to 0.65.
Genome-wide association studies (GWAS) have contributed to the discovery of many susceptibility variants. However, a large proportion of the heritability still remained unexplained. The "missing heritability" is probably hidden in the genome among the large number of variants with small effects [15].
Smoking and Alcohol Consumption
Smoking has been consistently linked with psoriasis. Studies that examined the exposure before the onset of psoriasis and control for confounding factors offer the more convincing evidence (Table 1) [16•, 17••, 18••, 19•, 20••]. In a combined analysis of three large cohorts in the United States, there was a trend toward an increased risk of psoriasis with increasing pack-years or duration of smoking. In addition, there was a graded reduction of risk with an increase in time since smoking cessation [20••]. Genetic-environmental interaction involving smoking also has been proposed even if not well documented by a few studies of psoriasis. In a study, a stronger association between smoking and psoriasis in subjects with nonvariant CYP1A1 genotype was presented, suggesting that sequence variation in genes coding for phase 1 and phase II enzymes, including members of cytochrome P450 (CYP) family, may alter individual susceptibility to developing psoriasis in smokers, similarly to the documented effects in cancer and coronary artery disease [21]. In another study from a Chinese group, the risk of psoriasis for smokers with HLA-Cw6 increased approximately 11-fold than nonsmokers without HLA-Cw6 [22]. In an additional study, evidence was presented that polymorphisms in the IL13/IL4 region (especially rs1800925*T) may associate with protection from developing psoriatic arthritis and that this effect may be abrogated by smoking. It was unclear, from the data presented, whether smoking per se was associated with psoriatic arthritis [23•]. Cigarette smoking is a complex risk factor that involves a cocktail of more than 4,000 chemicals [24]. Nicotine is the principal alkaloid in tobacco. It exerts its effects by activating various subtypes of nicotinic acetylcholine receptors (nAChRs), which are classically found in the nervous system and adrenal medulla but also have been identified in nonneuronal tissue, such as skin keratinocytes; nAChRs facilitate cell-to-cell communication, keratinocyte adhesion, and upward migration in the epidermis. Components of tobacco smoking other than nicotine, such as acrolein, benzo(a)pyrene, or hydroquinone, also may exert proinflammatory and vascular events. Not only the effects of smoking are due to complex actions of various substances, but, as confirmed in a recent meta-analysis, they also may be modulated by gender with females at higher risk compared with males for overall morbidity and mortality associated with smoking at low and high level of use [25]. Cigarette smoking is a risk factor for more than two dozen diseases and the single biggest cause of preventable mortality worldwide. Besides the well-known association with cardiovascular disease and several cancers, it appears to be associated with a number of inflammatory immune-related disease, and it has been strongly linked with palmoplantar pustolosis. These associations may, at least partly, explain selected comorbidities of psoriasis.
The evidence concerning the association of incident psoriasis with alcohol consumption is controversial, being partly confounded by smoking [26•]. The risk may vary depending on the type of alcoholic beverage considered. In a large-scale cohort study, nonlight beer intake was associated with an increased risk of developing psoriasis among women, whereas other alcoholic beverages, including light beer, wine, and spirits, did not increase the risk of the disease [27•]. The reasons for this specific association are unclear. A tentative explanation is that beer is one of the few nondistilled alcoholic beverages that use a starch-source for fermentation, i.e., barley. Barley contains gluten, and gluten sensitivity has been linked with psoriasis.
As documented in a meta-analysis, increased alcohol consumption is associated with prevalent psoriasis and, hence, it may represent a consequence of living with the disease [28•]. Smoking and alcohol may alter the expression of psoriasis (e.g., pattern distribution, clinical varieties) and its clinical course. Smoking has been linked with acral and pustular lesions. Alcohol has been associated with severity of psoriasis and treatment failures [26•].
Body Weight, Diet, and Physical Exercise
It is well established that increased body mass index (BMI) and increased waist circumference are risk factors for developing psoriasis. The association has been documented, in a consistent way, both in case-control studies of incident psoriasis cases and in cohort studies [17••, 19•, 29••]. Interestingly, the association also has been found in childhood-onset psoriasis (age 0-18 years) [30, 31•, 32–34] and in psoriatic arthritis [35•, 36••]. In the largest cohort study, body mass index (BMI) information was updated every 2 years. The risk of psoriasis almost doubled in people with BMI equal or higher than 35.0 compared with people with BMI 21.0-22.9; weight gain from the age of 18 years, higher waist circumference, hip circumference, and waist-hip ratio were all associated with a higher risk of incident psoriasis [29••]. Interestingly, a multinational cross-sectional survey (not a true analytic epidemiology study) of children from nine different countries documented a similar association over the countries, of prevalent psoriasis, regardless of disease severity, with increased BMI and increased central adiposity [34]. Obesity in childhood has been associated with increased metabolic risk and cardiovascular morbidity in adulthood, hence young psoriatic patients warrant early monitoring and lifestyle modification [37]. Interestingly, significant multiplicative interaction has been recently documented in adult psoriatic patients between BMI, waist circumference, and two SNPs in the IL12B (rs3212227) and IL23R genes (rs7530511) [38].
Overweight and obesity also has been linked with a reduced response to systemic treatment in a registry of severe psoriatic patients [39], whereas, in case series, bariatric surgery and weight loss were associated with a remarkable clinical improvement of psoriasis [40]. Obesity is a metabolic and inflammatory disorder. Adipokines, e.g., chemerin, are biomarkers of obesity-related inflammation. It has been documented that patients with psoriasis have higher blood levels of adipokines, which normalize during therapy [41].
Scanty data are available concerning the role of diet in psoriasis. In an Italian case-control study, the risk of psoriasis increased with increasing BMI and was inversely related to the consumption of carrots, tomatoes, and fresh fruit and to the index of beta-carotene intake [42]. An association between gluten sensitivity, celiac disease, and psoriasis has been proposed and, recently, confirmed by a large scale cohort study of 28,958 patients suffering from celiac disease and 143,910 sex- and age-matched controls, with an hazard ratio for psoriasis of 1.78 [43••]. The association also was confirmed in children. Interestingly, in a small study of 33 psoriatic patients with antigliadin antibodies, psoriasis improved on a gluten-free diet [44]. A recent cohort study documented that vigorous physical activity was independently associated with a reduced risk of incident psoriasis [45••].
Drugs and Infections
Several drugs, e.g., lithium salts, beta-adrenergic blocking agents, antimalarials, have been reported to be responsible for the onset or exacerbation of psoriasis but evidence, with the exception of lithium salts [46••], is limited or inconclusive [47••]. A possible protective effect on psoriasis has been reported for the use of atypical antipsychotics and the oral antidiabetics thiazolidinediones [46, 48•]. Paradoxical adverse effects, defined as the onset or exacerbation of disorders that are usually improved, have been reported with tumor necrosis factor (TNF)-alpha antagonists, including new psoriasis onset. The skin lesions develop within the first few months of therapy, and patients with a wide range of underlying diseases can be affected. Palmoplantar pustulosis also is a common feature. The prevalence of this adverse effect has been estimated at 1.5-5 % of patients taking TNF-alpha antagonists [49].
An infection with beta-hemolytic streptococci often precedes the first manifestation of guttate psoriasis with an odds ratio of 7.8 estimated in an Italian case-control study [50]. Furthermore, a cohort study in the United States, involving 265,000 members of the Harvard Community Health Plan, demonstrated that chronic HIV infection is linked to a higher risk of psoriasis (relative risk 3.5). The risk increases with the progression of the disease from the asymptomatic phase to full-blown AIDS [51].
Psychosocial Factors
Psychosomatic factors are deemed to play a role in psoriasis, and stressful life events have been linked with the risk of incident psoriasis. A major problem in this area is that virtually all of the research is based on the recall of past events. People have a strong tendency to seek explanations to account for what happens to them and stress is commonly used for this. Recently, the association between depression and the risk of new-onset psoriasis was analyzed in a prospective cohort study of 86,880 American female nurses (The Nurses' Health Study II. Participants reported antidepressant use and completed the Mental Health Index (MHI), a subscale of the Short-Form 36). Depression was associated with an increased risk of incident psoriasis (relative risk 1.59; 95 % confidence interval, 1.21-2.08) [52•].
Conclusions
Genetic-environmental interaction has been proposed as a model for the causation of psoriasis. Environmental risk factors that have been proposed include smoking, alcohol consumption, diet, overweight and physical inactivity, infection, drugs, and stressful life events. Not all of them have been adequately documented in epidemiological studies. The best documented are smoking and obesity. By imposing methodologic control and a numerate approach, epidemiology can offer a major contribution to understand the causation of psoriasis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kannel WB, Dawber TR, Kagan A, Revotskie N, Stoke 3rd J. Factors of risk in the development of coronary heart disease—six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50.
Bradford-Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300.
Shing YY. The prevalence of psoriasis in the Mongoloid race. J Am Acad Dermatol. 1984;10:965–8.
•• Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; on behalf of the Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2012 Sep 27. doi:10.1038/jid.2012.339. [Epub ahead of print]. A systematic review of psoriasis prevalence and incidence data worldwide. An essential reference paper.
• Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–41. A paper exploiting data from the UK General Practice Research Database showing that the prevalence of psoriasis declines significantly in patients 70 years and older, possibly due to increased mortality rates in older psoriatic patients compared with the general population of the same age.
Duffy DL, Spelman LS, Martin NG. Psoriasis in Australian twins. J Am Acad Dermatol. 1993;29:428–34.
Braathen LR, Botten G, Bjerkedal T. Prevalence of psoriasis in Norway. Acta Derm Venereol. 1989;142(suppl):5–8.
Kavli G, Førde OH, Arnesen E, Stenvold SE. Psoriasis: familial predisposition and environmental factors. Br Med J. 1985;291:999–1000.
Khoury MJ, Beaty TH, Cohen BH. Fundamentals of genetic epidemiology. New York: Oxford University Press; 1993.
Armenian HK, Khoury MJ. Age at onset of genetic diseases: an application of Sartwell's model for the distribution of the incubation period. Am J Epidemiol. 1981;113:596–605.
Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13:450–6.
•• Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Kremers HM. Trends in incidence of adult-onset psoriasis over three decades: a population based study. J Am Acad Dermatol. 2009;60:394–401. Two related papers (ref. 12 and 13) from the same research group based on data resources of the Rochester Epidemiology Project in the Olmsted County, Minnesota. Validated CASPAR criteria were used for the diagnosis of psoriatic arthritis. The two studies document a trend toward increasing incidence of both psoriasis and psoriatic arthritis from January 1970 to December 1999. The annual incidence rate of psoriasis almost doubled during the study period.
•• Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over three decades: A population-based study. J Rheumatol. 2009;36:361–7. Two related papers (ref. 12 and 13) from the same research group based on data resources of the Rochester Epidemiology Project in the Olmsted County, Minnesota. Validated CASPAR criteria were used for the diagnosis of psoriatic arthritis. The two studies document a trend toward increasing incidence of both psoriasis and psoriatic arthritis from January 1970 to December 1999. The annual incidence rate of psoriasis almost doubled during the study period.
Brandrup F, Holm N, Grunnet N, Henningsen K, Hansen HE. Psoriasis in monozygotic twins: variations in expression in individuals with identical genetic constitution. Acta Derm Venereol. 1982;62:229–36.
Oka A, Mabuchi T, Ozawa A, Inoko H. Current understanding of human genetics and genetic analysis of psoriasis. J Dermatol. 2012;39:231–41.
• Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143:1559–65. A prospective cohort study with nested case-control analysis using the U.K. General Practice Research Database containing computerized clinical information entered by general practitioners. The incidence rate of psoriasis was 14 per 10,000 person-year. Patients with antecedents of skin disorders and skin infection within the last year had the highest risk of developing psoriasis (misclassified prodromal signs of psoriasis?). Also, smoking was found to be an independent risk factor. No association was documented with antecedents of stress, diabetes, hypertension, hyperlipidemia, cardiovascular disease, or rheumatoid arthritis.
•• Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61–7. An Italian study part of a long term case-control surveillance programme of newly diagnosed cases of psoriasis with history of skin manifestations no longer than one year. Several purported risk factors were simultaneously assessed.
•• Setty AR, Curhan G, Choi HK. Smoking and the risk of psoriasis in women: Nurses’ Health Study II. Am J Med. 2007;120:953–9. The Nurses’ Health Studies are among the largest and longest running investigations of factors that influence women’s health. Started in 1976 and expanded in 1989, information has provided by about 238,000 dedicated nurse-participants ( http://www.channing.harvard.edu/nhs/ ). The relationship between smoking status (including duration, intensity, cessation, and exposure to secondhand smoke) and incident psoriasis was examined during a 14-year time period (1991-2005). The primary outcome was incident, self-reported, physician-diagnosed psoriasis.
• Wolk K, Mallbris L, Larsson P, Rosenblad A, Vingård E, Ståhle M. Excessive body weight and smoking associates with a high risk of onset of plaque psoriasis. Acta Derm Venereol. 2009;89:492–7. A population-based case-control study from Sweden, including 373 cases with onset of first-time plaque psoriasis within 12 months and matched healthy controls. The study confirms that smoking and obesity are risk factors for psoriasis.
•• Li W, Han J, Choi HK, Qureshi AA. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol. 2012;175:402–13. The study presents a combined analysis of three cohorts: a cohort of older women (the Nurses' Health Study, 1996-2008), a cohort of younger women (the Nurses' Health Study II, 1991-2005), and a cohort of men (Health Professionals' Follow-up Study, 1986-2006). A total of 185,836 participants (2,410 with incident psoriasis) were included. Information on smoking was collected biennially during follow-up. The relative risk of incident psoriasis was 1.94 (95 % CI: 1.64, 2.28) for current smokers. There was a graded reduction of risk with an increase in time since smoking cessation.
Jin Y, Yang S, Zhang F, Kong Y, Xiao F, Hou Y. Combined effects of HLA-Cw6 and cigarette smoking in psoriasis vulgaris: a hospital-based case-control study in China. J Eur Acad Dermatol Venereol. 2009;23:132–7.
Krämer U, Esser C. Cigarette smoking, metabolic gene polymorphism, and psoriasis. J Invest Dermatol. 2006;126:693–4.
• Duffin KC, Freeny IC, Schrodi SJ, Wong B, Feng BJ, Soltani-Arabshahi R, et al. Association between IL13 polymorphisms and psoriatic arthritis is modified by smoking. J Invest Dermatol. 2009;129:2777–83. This paper presents evidence that polymorphisms in the IL13/IL4 region may associate with protection from developing psoriatic arthritis and that this effect is abrogated by smoking. It is unclear from the data presented whether smoking per se associated with psoriatic arthritis.
Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–7.
Mucha L, Stephenson J, Morandi N, Dirani R. Meta-analysis of disease risk associated with smoking, by gender and intensity of smoking. Gend Med. 2006;3:279–91.
• Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:121–8. A review of epidemiologic data on psoriasis including descriptive, analytic and clinical epidemiology.
• Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol intake and risk of incident psoriasis in U.S. women: a prospective study. Arch Dermatol. 2010;146:1364–9. A paper based on data from the Nurses’ Health Study II. Nonlight beer intake was associated with an increased risk of developing psoriasis among women. Other alcoholic beverages did not increase the risk.
• Zhu KJ, Zhu CY, Fan YM. Alcohol consumption and psoriatic risk: a meta-analysis of case-control studies. J Dermatol. 2012;39:770–3. A meta-analysis of 15 case-control studies. Most of the studies were actually cross-sectional and based on prevalent cases.
•• Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women. Nurses’ Health Study II. Arch Intern Med. 2007;167:1670–5. The relationships between BMI, weight change, waist circumference, hip circumference, waist-hip ratio, and incident psoriasis were analyzed over a 14-year period in 78,626 women participating in the Nurses' Health Study II. There were 892 self-reported incident cases of psoriasis. A graded positive association between BMI measured at multiple time points and the risk of incident psoriasis was documented. Weight gain from the age of 18 years, higher waist circumference, hip circumference, and waist-hip ratio were associated with psoriasis.
Bryld LE, Sørensen TI, Andersen KK, Jemec GB, Baker JL. High body mass index in adolescent girls precedes psoriasis hospitalization. Acta Derm Venereol. 2010;90:488–93.
• Ozden MG, Tekin NS, Gürer MA, et al. Environmental risk factors in pediatric psoriasis: a multicenter case-control study. Pediatr Dermatol. 2011;28:306–12. A multicenter, case-control study of 537 patients with psoriasis and 511 controls younger than age 18 years. Overall, patients more frequently reported exposure to environmental tobacco smoke at home (OR 2.9) and stressful life events in the year preceding the diagnosis than controls (OR 2.9). In addition, children with psoriasis were more likely to have a higher BMI (>26) than controls (OR = 2.5).
Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484–6.
Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162:633–6.
Paller AS, Mercy K, Kwasny MJ, Choon SE, Cordoro KM, Girolomoni G, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. Arch Dermatol. 2012;19:1–11. doi:10.1001/jamadermatol.2013.1078 [Epub ahead of print].
• Soltani-Arabshahi R, Wong B, Feng BJ, Goldgar DE, Duffin KC, Krueger GG. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol. 2010;146:721–6. A study in 943 volunteering patients with dermatologist-diagnosed psoriasis enrolled in the Utah Psoriasis Initiative (2002-2008). BMI at age 18 years was predictive of psoriatic arthritis. Koebner phenomenon and nail involvement were also associated with psoriatic arthritis.
•• Love TJ, Zhu Y, Zhang Y, Wall-Burns L, Ogdie A, Gelfand JM, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71:1273–7. A cohort study using data from The Health Improvement Network, a medical records database representative of the UK general population, collected between 1995 and 2010. The exposure of interest was the first BMI measured after psoriasis diagnosis and endpoints were incident cases of physician-diagnosed psoriatic arthritis. Among 75,395 individuals with psoriasis, 976 developed psoriatic arthritis (incidence rate, 26.5 per 10,000 person-years). The incidence rates increased with increasing BMI.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85.
Li WQ, Han JL, Zhang MF, Qureshi AA. Interactions between adiposity and genetic polymorphisms on the risk of psoriasis. Br J Dermatol. 2012. doi:10.1111/bjd.12001 [Epub ahead of print].
Naldi L, Addis A, Chimenti S, Giannetti A, Picardo M, Tomino C, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology. 2008;217:365–73.
Hossler EW, Wood GC, Still CD, Mowad CM, Maroon MS. The effect of weight loss surgery on the severity of psoriasis. Br J Dermatol. 2012. doi:10.1111/j.1365-2133.2012.11211.x [Epub ahead of print].
Gisondi P, Lora V, Bonauguri C, Russo A, Lippi G, Girolomoni G. Serum chemerin is increased in patients with chronic plaque psoriasis and normalizes following treatment with infliximab. Br J Dermatol. 2012. doi:10.1111/bjd.12118 [Epub ahead of print].
Naldi L, Parazzini F, Peli L, et al. Dietary factors and risk of psoriasis. Results of an Italian case-control study. Br J Dermatol. 1996;134:101–6.
•• Ludvigsson JF, Lindelöf B, Zingone F, Ciacci C. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010–6. Through 28 pathology departments in Sweden, 28,958 cases of celiac disease diagnosed between 1969 and 2008 were identified and compared with 143,910 sex- and age-matched controls regarding their risk of psoriasis. Celiac disease was a risk factor for future psoriasis (HR = 1.72). The same positive association was documented in children (HR = 2.05). The association was independent of a temporal relationship, because a positive association between celiac disease and psoriasis also was documented before the diagnosis of celiac disease was made.
Michaëlsson G, Gerdén B, Hagforsen E, Nilsson B, Pihl-Lundin I, Kraaz W, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44–51.
•• Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918–24. A study based on data from the Nurses' Health Study II. Women completed detailed physical activity questionnaires in 1991, 1997, and 2001. The risk of self-reported diagnosis of psoriasis by quintile of physical activity was calculated. The most physically active quintile of women had a lower risk of psoriasis compared with the least active quintile (RR 0.72). Vigorous physical activity (≥6 metabolic equivalents) also was associated with a reduced risk of psoriasis (RR 0.66).
•• Brauchli YB, Jick SS, Curtin F, Meier CR. Lithium, antipsychotics, and risk of psoriasis. J Clin Psychopharmacol. 2009;29:134–40. A case-control analysis using data from the UK General Practice Research Database. A total of 36,702 incident cases of psoriasis and the same number of matched controls were identified. Long-term use of lithium was associated with a small increase in risk of incident psoriasis (ORs of 1.68). There was a suggestion of a possible reduced psoriasis risk associated with the use of atypical antipsychotics, mainly olanzapine.
•• Brauchli YB, Jick SS, Curtin F, Meier CR. Association between beta-blockers, other antihypertensive drugs and psoriasis: population-based case-control study. Br J Dermatol. 2008;158:1299–307. A case-control analysis on the U.K. General Practice Research Database, encompassing 36,702 cases with a first-time psoriasis diagnosis and the same number of matched controls. The study did not support the current proposition that beta-blocker use is associated with an increased risk of psoriasis.
• Brauchli YB, Jick SS, Curtin F, Meier CR. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: A population based case-control study. J Am Acad Dermatol. 2008;58:421–9. A case-control analysis on the U.K. General Practice Research Database. Patients with incident psoriasis diagnosis were identified from 1994 to 2005 and matched to controls. Compared with no use, the OR for current use of five or more prescriptions for thiazolidinediones was 0.33 providing some further evidence for a potentially beneficial effect of thiazolidinediones on psoriasis. Also, metformin was associated with a suggestion of a reduced psoriasis risk.
Wendling D, Balblanc JC, Briançon D, Brousse A, Lohse A, Deprez P, et al. Onset or exacerbation of cutaneous psoriasis during TNFalpha antagonist therapy. Joint Bone Spine. 2008;75:315–8.
Naldi L, Peli L, Parazzini F, Carrel CF, Psoriasis Study Group of the Italian Group for Epidemiological Research in Dermatology. Family history of psoriasis, stressful life events, and recent infectious disease are risk factors for a first episode of acute guttate psoriasis: results of a case-control study. J Am Acad Dermatol. 2001;44:433–8.
Coopman SA, Johnson RA, Platt R, Stern RS. Cutaneous disease and drug reactions in HIV infection. N Engl J Med. 1993;328:1670–4.
• Dominguez PL, Han J, Li T, Ascherio A, Qureshi AA. Depression and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2012. doi:10.1111/j.1468-3083.2012.04703.x. [Epub ahead of print] An analysis conducted within the cohort of U.S. female nurses of The Nurses' Health Study II, followed up from 1993 to 2005. Participants reported anti-depressant use and completed the Mental Health Index (MHI), a subscale of the Short-Form 36 in 1993. Depression was associated with an increased risk of incident psoriasis.
Conflicts of interest
Dr. Naldi has received consultation fees from Novartis, Lilly, Pfitzer, Boehringer Ingelheim, and Amgen. He is an advisor for Janssen services, LLC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naldi, L. Risk Factors for Psoriasis. Curr Derm Rep 2, 58–65 (2013). https://doi.org/10.1007/s13671-012-0034-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-012-0034-6