Abstract
Purpose of Review
This paper provides an update of the recent evidence related to transcervical fibroid ablation (TFA) with the Sonata® System.
Recent Findings
An additional 27 papers representing over 400 women and more than 800 treated fibroids have been published. These demonstrate consistent, positive results, representing multiple prospective clinical trials, subgroup analyses, health economic analyses, case series, and systematic reviews of TFA. These include a 147-patient prospective clinical trial of TFA (the SONATA Clinical Trial) that demonstrated sustained symptom relief and an 8.2% cumulative reintervention rate through 3 years, a long-term study (VITALITY) confirming durable symptom relief with an 11.8% reintervention rate over > 5 years of mean follow-up, a clinical trial (OPEN) suggesting minimal potential for intrauterine adhesiogenesis post-TFA, preliminary results of a global registry (SAGE), and two subgroup analyses of TFA reporting favorable and safe outcomes in women with large fibroids > 5 cm in diameter. Three comparative health economic studies demonstrate favorable economic outcomes against both myomectomy and hysterectomy. A recent ACOG Practice Bulletin also noted equivalent outcomes for transcervical, laparoscopic, and transvaginal fibroid ablation.
Summary
Accumulated clinical evidence, including systematic reviews and longitudinal prospective clinical trials, continues to confirm the safety and efficacy of TFA in women with symptomatic fibroids, including myomata > 5 cm. As a transcervical treatment modality that can safely address all nonpedunculated uterine fibroid types, the continued evidence base confirms TFA as an innovative and useful treatment option that meets a significant unmet clinical need, including among underserved populations, delivering significant durable reductions in fibroid symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine fibroids are benign growths of smooth muscle within the uterus. They can be associated with significant negative effects upon a woman’s quality of life due to symptoms that include abnormal uterine bleeding, dyspareunia, abdominopelvic discomfort, and frequent urination. While curative, hysterectomy in women with symptomatic uterine fibroids results in loss of fertility and involves major surgery. Myomectomy, while conserving the uterus, is also a major surgical procedure when performed transperitoneally and can be associated with uterine rupture in future pregnancy [1,2,3]. While hysteroscopic myomectomy has the benefit of being an outpatient transcervical procedure, it is limited to the treatment of submucous fibroids as only these may be readily accessed through the hysteroscope. Rarely, uterine rupture has been reported after hysteroscopic myomectomy [4].
There are significant unmet needs regarding fibroid treatment. These include a need for better diagnostic options, improved access to care, a definition of best practices, better treatment options that conserve the uterus and fertility, patient empowerment, and education [5]. In recent years, there has been increasing awareness of disparities in care among women of color in the USA. It has been established that Black women have a higher prevalence of uterine fibroids, yet undergo lower rates of minimally invasive procedures than White women [6•, 7]. Multiple investigations have indicated that Black women are more likely than White women to undergo surgical treatments for symptomatic uterine fibroids, and that Black women have a significantly higher rate of hysterectomy and its complications than White women; this is especially disparate in counties with higher socioeconomic status [8–11]. For all these reasons, it is important that innovative, less invasive treatment options for uterine fibroids are developed, evaluated, and made available to women to enhance access to necessary care.
Transcervical fibroid ablation (TFA) continues to gain momentum as an ideal treatment of uterine fibroids. This is a focal, volumetric image-guided radiofrequency procedure that enables the optimized ablation of uterine fibroids, does not involve the peritoneal cavity or uterine serosa, and incites coagulative necrosis; this results in significantly reduced symptoms, while conserving the uterus in a safe outpatient manner [12•, 13••]. A 2017 review of TFA provided clinical data from a 50-patient study (FAST-EU) at seven sites in Europe (6) and Mexico (1) [14••]. Eighty-nine fibroids were treated in that study with TFA, with mean reductions in fibroid volume of 68.1% ± 28.6 and 67.4% ± 31.9 at 3 and 12 months, respectively (P < 0.001 compared with baseline; Wilcoxon signed-rank test). There were significant improvements in symptom severity and health-related quality of life (HRQOL) at 3, 6, and 12 months post-ablation.
Since that review, 27 papers have been published describing consistent and positive results for TFA with the Sonata System, including multiple prospective clinical trials, subgroup analyses, health economic analyses, case series, and systematic reviews of TFA. These publications include the 147-patient SONATA trial, the 37-patient OPEN trial, the 160-patient SAGE registry, a 50-patient case series describing TFA outcomes for women with large fibroids, and 19 additional unique patients captured in a pregnancy case series. In combination with the FAST-EU trial, the literature base represents 463 women in total with over 900 treated fibroids. This updated review will provide additional clinical evidence confirming the safety and effectiveness of TFA. As the use of RF energy to ablate fibroids becomes more widespread, it will be important to understand where TFA fits in the evolving paradigm for fibroid care.
Transcervical Fibroid Ablation (TFA) and the Sonata® System
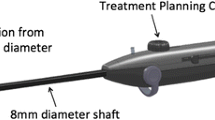
TFA is an ambulatory fibroid ablation procedure provided by the Sonata® System (Gynesonics; Redwood City, CA), which is a FDA-cleared and CE-marked device and currently the only commercially available device for TFA (Fig. 1) [15•]. Because the Sonata device integrates intrauterine sonography with the delivery of RF energy in a single device, the ultrasound beam is always within the plane of the needle electrode array, facilitating the ability to appropriately target and ablate fibroids in a safe and straightforward manner [16, 17••]. The combination of intrauterine sonography with an RF ablation component enables the treatment of a broader range of uterine fibroids than can be resected hysteroscopically.
The intrauterine ultrasound (IUUS) probe within the Sonata device has a center frequency of 7.5 MHz and a depth of penetration > 9 cm, with a 114° field of view. A typical IUUS image is shown in Fig. 2. The Sonata System can place ablations in all nonpedunculated uterine fibroids; type 0, type 7, and type 8 (cervical and other extrauterine fibroids) are not recommended for treatment with Sonata [15•, 18•]. Depending on the ablation size, which is determined graphically by the gynecologist user, single ablation times range from 1 to 7 min, and multiple overlapping ablations may be performed in one or more fibroids during the same treatment session [12•, 19, 20].
The system provides a real-time ultrasound display enabling visualization of fibroids with a graphical targeting guide overlaying the image. This overlay depicts the location and size of the subsequent ablation zone (red ellipsoid), along with a thermal safety border (green ellipsoid) that demarcates a point beyond which there are no significant thermal effects on tissue (Fig. 3) [15•]. The use of a validated safety border, in conjunction with a depiction of the ablation size and location, provides a focused and precise mechanism for creating ablations safely without extending beyond the uterine serosa, preventing damage to the surrounding viscera. Ablations may also be limited to stay within a fibroid’s pseudocapsule if desired by the operator.
The Sonata SMART Guide, demonstrating the ablation zone (red ellipsoid) and thermal safety border (green ellipsoid) and uterine serosa along with a graphic of the tip of the Sonata IUUS Probe. The ablation zone depicts the size and location of the thermal ablation as chosen graphically (in real time) by the gynecologist performing TFA. The thermal safety border represents the border beyond which tissue is safe from potential thermal damage
As with any fibroid treatment, including hysterectomy and myomectomy, gynecologists should keep in mind the potential for uterine malignancy, including adenocarcinoma and sarcoma, especially if contemplating treatment of postmenopausal women. Endometrial sampling should be considered when appropriate (e.g., women > 45 years of age with abnormal uterine bleeding), along with biopsy if any solid lesions appear suspicious on imaging. That said, TFA is not an intraperitoneal fibroid treatment, nor does it involve resection or power morcellation.
The SONATA Clinical Trial
The sonography-guided transcervical ablation of uterine fibroids (SONATA) pivotal trial was a 147-patient prospective, interventional, multicenter, single-arm FDA trial in which 147 women with symptomatic fibroids were enrolled and treated at 22 sites (21 in the USA and one in Mexico) and followed through 36 months post-ablation [13••]. The co-primary endpoints, both measured at 12 months, were reduction in menstrual blood loss (MBL) and the rate of surgical reintervention for heavy menstrual bleeding (HMB). A variety of secondary endpoints, including patient satisfaction, return to normal activity, safety, and the symptom severity score (SSS) and health-related quality of life (HRQOL) subscales of the Uterine Fibroid Symptom-Quality of Life (UFS-QOL) questionnaire, were also reported. Premenopausal women 25–50 years of age were included if they had 1–10 treatable fibroids (FIGO type 1, type 2, type 3, type 4, type 5, type 6, and hybrid type 2–5) up to 5 cm in maximal diameter based on baseline transvaginal sonography; at least one fibroid was required to be indenting the endometrial cavity (type 1, type 2, type 2–5, or abutting type 3). As this was a bleeding study, women were required to have a minimum baseline pictorial blood loss assessment chart (PBAC) score between 150 and 500 as well as predictable, consistent menstrual cycles. Women were excluded for several factors, including if they desired pregnancy, had clinically significant adenomyosis as determined by baseline MR imaging, or had prior endometrial ablation or uterine artery embolization (UAE).
The mean age of patients enrolled in SONATA was 43 years. The study population was noteworthy for its diversity (33.3% of the patients were Black, 29.3% were Latina). In total, 442 fibroids were ablated among the 147 patients, and the mean length of stay was 2.5 h (inclusive of the procedure time). Anesthesia choice was determined by individual physicians; 50.3% of patients received general anesthesia while 49.7% received conscious sedation, with mean recovery pain scores of 3.4/10 and 1.9/10, respectively. Patients were treated in all three sites of service as outpatients: hospital operating rooms, ambulatory surgery centers, and physician offices [13••].
By 3 months post-ablation, there was a mean 39% reduction in menstrual bleeding as assessed by the PBAC (P < 0.001 from baseline) and menstrual bleeding was further reduced to 51% at 12 months (P < 0.001 from baseline). By 12 months, 95% of women reported a reduction in menstrual bleeding, with 65% of women reporting at least a 50% reduction in menstrual bleeding (P < 0.001 from baseline). Ninety-seven percent of women were satisfied with their treatment at 12 months. Transcervical fibroid ablation with the Sonata System was also associated with cumulative rates of surgical reintervention for HMB of 0.7%, 5.0%, and 8.2% at 1, 2, and 3 years, respectively (Kaplan–Meier estimator method) [21••]. Patient-reported outcomes for the UFS-QOL questionnaire demonstrated statistically significant improvements in both the SSS and HRQOL subscales at 3 months and these improvements were sustained through 3 years (Figs. 4 and 5) [13••, 21••].
On average, women returned to normal activity within 2.2 days, with half returning to normal activity within 1 day [13••]. With 132 out of 147 treated patients (90%) accounted for at 3 years, 94% noted satisfaction with their treatment, and absenteeism from work due to fibroid symptoms decreased by > 50% (P < 0.001) [21••].
Regarding safety outcomes, there were no device-related adverse events. Two procedure-related serious adverse events were identified in the first year of follow-up and resolved without sequelae. These consisted of a deep venous thrombosis at 15 days post-ablation that was managed with oral anticoagulants on an outpatient basis, and an overnight admission for noninfectious leukorrhea and pelvic pain 28 days after TFA. The three most frequent nonserious procedure-related adverse events consisted of fibroid sloughing (30.6%), cramping/pain (7.5%), and leukorrhea (6.1%). Fibroid expulsion was noted in 1.4% of patients through 12 months [13••].
Two pregnancies resulted over the 3-year follow-up period [21••]. One patient conceived 22 months post-TFA, and her pregnancy culminated in an elective repeat Cesarean section at 38 2/7 weeks’ gestation. At 29 months post-TFA, a 40-year-old subject reported a first-trimester pregnancy loss.
Long-Term Clinical Outcomes of Transcervical Radiofrequency Ablation of Uterine Fibroids: the VITALITY Study
VITALITY was a retrospective, single center, single-arm clinical study that involved longer-term follow-up of a 17-patient cohort from the 12-month FAST-EU trial of TFA that had been conducted at 7 sites in Europe and Mexico [14••, 17••]. Of note, there were no surgical reinterventions for HMB within the first 3.5 years status-post TFA, and an 11.8% cumulative reintervention rate over the mean follow-up period of 64 months (range: 57–73 months). Significant improvements in the SSS and HRQOL subscales of the UFS-QOL questionnaire remained durable through that mean follow-up of 64 months (Fig. 5).
The OPEN Clinical Trial
Yang and colleagues have noted a 1.5–2.4% risk of intrauterine adhesiogenesis 1–3 months after hysteroscopic myomectomy of solitary fibroids, with a 78% incidence after resection of ≥ 2 apposing fibroids [22]. The OPEN clinical trial was a prospective, multicenter, single-arm, observational study to investigate the incidence of new intrauterine adhesions following TFA [23••]. The primary endpoint was the incidence of new intrauterine adhesions at 6 weeks post-treatment.
Patients were enrolled if they had symptomatic fibroids and expressed a desire to undergo TFA with the Sonata System at any of the six sites located in Germany, Switzerland, and the UK; had at least one fibroid that indented the endometrial cavity (e.g., FIGO type 1, type 2, type 2–5) without limitations on size or number of fibroids being treated; and were willing to undergo second-look hysteroscopy at 6 weeks post-ablation. No adhesion barriers were permitted. Videos of baseline and second-look hysteroscopies were reviewed by an independent committee and, if present, any adhesions were scored per the European Society of Hysteroscopy system [24]. Patients with preexisting intrauterine synechiae at baseline, as evaluated by the independent committee, were excluded.
Of 37 women with an aggregate 50 fibroids who were enrolled and treated in OPEN, two patients withdrew after treatment without returning for a second-look hysteroscopy, while one patient’s hysteroscopy video was not evaluable. Among the 34 treated women who had paired baseline and second-look hysteroscopy videos that could be evaluated by the committee, no intrauterine adhesiogenesis was noted, including among six patients with apposing fibroids. The authors concluded “These results suggest the potential for adhesiogenesis after transcervical fibroid ablation, including in women with apposing submucous and/or transmural myomata, may be minimal.”
Transcervical Fibroid Ablation for Fibroids > 5 cm in Maximal Diameter
Shifrin and colleagues performed a subgroup analysis of patients from both the FAST-EU and SONATA Clinical Trials with large fibroids > 5 cm [18•]. Of 50 women treated in FAST-EU, 10 (20%) had at least one fibroid > 5 cm. This represented 12 fibroids in total (13% of 92 ablated fibroids), with the largest treated fibroid having been 6.9 cm in maximal diameter. In SONATA (total n = 147), there were 9 women with a total of 11 large fibroids (2.5% of 442 treated fibroids), the largest myoma having a diameter of 6.5 cm. Reductions in menstrual bleeding and improvements in SSS and HRQOL in these subgroups were significant beginning at 3 months post-ablation and were comparable to those seen for the overall cohort in both studies and were sustained over 12 months. There was a mean reduction in large (> 5 cm) fibroid volume of 68.3% at 12 months in the FAST-EU trial, similar to the overall cohort’s mean 12-month fibroid volume reduction of 66.6% [14••, 18•].
Piriyev and colleagues retrospectively analyzed 151 patients treated on a post market basis in their department over a 10-year period and reported on the outcomes of 50 women with at least one fibroid ≥ 5 cm [25•]. All patients were treated for HMB. There were 57 fibroids treated, ranging in size from 4 to 12 cm, and the maximum number of ablations performed was five, in a single treatment session. Overall, 86% of their patients with ablated fibroids ≥ 5 cm noted symptom improvement. The authors reported an absence of intraoperative or postoperative complications and no reinterventions were noted. They concluded “Thus, the Sonata® System is a simple, minimally invasive, rapid, and successful method that shows significant improvement of symptoms even in large myomas from ≥ 5 cm” [25•].
The SAGE Clinical Registry
SAGE is a global post market registry, aiming to characterize long-term outcomes and real-world use experience following TFA for treatment of symptomatic uterine fibroids. The registry is ongoing and will enroll up to 500 women at up to 50 sites worldwide [26•]. The primary outcomes include the SSS and HRQOL subscales of the UFS-QOL, health status as indicated by the EQ-5D questionnaire, patient satisfaction, adverse events, pregnancy occurrence and outcomes, and surgical reintervention for HMB. Patients enrolled in the registry are followed 1-month post-ablation and then yearly through 5 years. The only inclusion criteria are age (18 years and older), the presence of symptomatic uterine fibroids, a desire to participate in the registry, and selection of TFA as the primary treatment option. To date, women in the registry have been treated at 8 sites in Germany, Switzerland, and the UK. The following is a summary from the most recent publication on this registry.
Preliminary results of the first 160 patients enrolled in SAGE of TFA outcomes were published by Christoffel and colleagues [26•]. Mean age was 42 ± 7 years, and the mean follow-up was 5.3 ± 6 months (maximum follow-up was 25 months). In all, 241 fibroids were ablated, ranging in size from < 1 to > 10 cm, with 27% having diameters > 5 cm. The range of treated fibroids included submucous (10%), transmural (52%), intramural (28%), and subserous myomata (10%).
As with earlier clinical studies, there were no serious device-related adverse events. There was one case of endometritis reported (0.6%) that was deemed a serious procedure-related adverse event. A second-degree skin burn at the site of the dispersive electrode, resulting from human error/misuse by site staff, was reported and classified as device-related (0.6%).
Health Economic Studies
Several health economic studies and analyses have been performed for TFA with the Sonata System. A paper by Huirne and Brooks noted significant improvements in health utility from baseline to 12 months in 49 women treated as part of the earlier FAST-EU trial [27•]. There is a similar health economic analysis of 147 women from the SONATA Clinical Trial demonstrating durable improvements over 3 years in generic and fibroid-specific quality of life, along with clinically meaningful increases in quality-adjusted life years (QALYs) [28•].
There are three recent studies examining surgical economic cost comparisons that involve patients treated as part of the SONATA Clinical Trial. The COMPARE study was a cost analysis of hospital charge data from the perioperative through 30-day follow-up period, compared against that of myomectomy and hysterectomy [29•]. Patients undergoing TFA had a significantly lower mean length of stay (5.1 h) compared against both hysterectomy (73 h) and myomectomy (80 h) (all P < 0.001). From a facility perspective, this led to a significantly lower cost for TFA procedures ($7701) vs hysterectomy ($10,353) and myomectomy ($12,003).
Similarly, the INSPIRE study examined perioperative and 1-year cost and health care resource utilization (HCRU) data for TFA, myomectomy, and hysterectomy from a payor perspective [30•]. INSPIRE found that use of the Sonata System to perform TFA was associated with significantly reduced index procedure, complication, and admission costs through 12 months compared to myomectomy and hysterectomy. Besides a reduced length of stay, patients undergoing TFA had significantly lower payor costs for complications, imaging, and prescription medications than did myomectomy and hysterectomy.
A facility-level comparative study of TFA was performed (the CHOICES study) involving 88 women equally divided into a TFA cohort (n = 44) and myomectomy (n = 44) who were case-matched retrospectively, with treatment having been provided at four centers [31•]. The CHOICES study found that TFA had a significantly reduced mean operating room duration and length of stay than myomectomy. Mean procedure, anesthesia, laboratory, pathology, and pharmacy costs were significantly lower for TFA compared to myomectomy. When stratified by route and site of service, TFA was associated with significantly lower mean procedure costs compared to inpatient, abdominal, or laparoscopic myomectomy.
Systematic Reviews of TFA/Clinical Guidelines
Three systematic reviews involving TFA have been published to date. The first, by Taheri and colleagues, reviewed reductions in uterine and fibroid volumes for nonresective fibroid treatments such as UAE, FUS, and RF ablation [32]. Their review included 81 papers representing RF ablation (n = 11), UAE (n = 52), and FUS (n = 17). The use of RF ablation, including TFA, was associated with the greatest overall percentage of fibroid volume reduction (70%) vs UAE (54%) and FUS (32%).
Bradley, Pasic, and Miller performed a systematic review and meta-analysis regarding the clinical performance of RF fibroid ablation (transcervical, transvaginal, and laparoscopic), involving 32 papers representing 1283 patients [33•]. Five papers involved TFA, specifically with the Sonata System. The authors concluded RF ablation was effective in significantly reducing fibroid volume and produces durable symptom relief and a low reintervention rate. Specific to TFA, the systematic review noted that a transcervical approach to fibroid ablation was associated across the five papers with a 2.5-h mean length of stay, a mean 3.3-day return to normal activity, and a 3.6-day mean return to work, all of which were less than those associated with operative laparoscopy (10.7 h, 9.0 days, and 6.5 days, respectively).
A recent systematic review specific to TFA with the Sonata System reviewed 10 studies involving 227 patients [34••]. The review examined reductions in bleeding and improvements in SSS and HRQOL as well as the overall reintervention rate. Synthesizing the studies (SONATA, FAST-EU, VITALITY, OPEN) along with three case reports, the authors concluded “Radiofrequency ablation with the Sonata System represents a minimally invasive, organ-preserving treatment option in patients with symptomatic uterine myomas, associated with clinically meaningful improvement of myoma-related symptoms.”
Gynecologists in Germany, Switzerland, and Austria convened in 2020 to develop consensus guidelines for TFA [35, 36, 37•]. They stated that TFA is preferable for treating FIGO type 2, type 3, type 4, and type 2–5 fibroids as well as an option for large type 1 fibroids, multiple fibroids, and any fibroids that are challenging to access using transperitoneal surgical treatment. They noted that TFA is associated with faster recovery compared with non-transcervical fibroid treatment options, as well as low rates of complications and surgical reintervention.
Finally, The American College of Obstetricians and Gynecologists updated its Practice Bulletin #228 on the Management of Symptomatic Uterine Leiomyomas and indicated that RF ablation is a “reasonable option” for the management of symptomatic fibroids [38]. The ACOG Practice Bulletin also noted equivalent outcomes for transcervical, laparoscopic, and transvaginal fibroid ablation.
Pregnancy after TFA
While the safety, optimal conception timing, and appropriate routes of delivery after TFA remain to be established, there have been pregnancies reported in the literature after TFA, including vaginal deliveries as well as after assisted reproduction (ART) [39, 40•, 41, 42].
Christoffel and colleagues recently reviewed a series of 36 pregnancy outcomes among 28 women who had undergone TFA for symptomatic uterine fibroids with the Sonata System [40•]. Of the 28 women, 9 had been enrolled in either FAST-EU, SONATA, or SAGE, while 19 were treated with TFA on a post market basis. There were 20 deliveries, 8 of which were achieved vaginally while 12 were via Cesarean section (C/S; 9 primary, 3 repeat). There were 3 therapeutic abortions and 8 spontaneous abortions (4 of which were in the same patient with apparent antiphospholipid syndrome). There were no low 5-min Apgar scores, no birth weight was < 2500 g, and all but one delivery (at 35 6/7 weeks) took place at or beyond 37 weeks’ gestation. There were no stillbirths or abnormal placentation, nor were postpartum hemorrhage or uterine rupture noted in the peripartum period. Five women reported more than one pregnancy after TFA, and 4 women conceived after ART. The largest ablated fibroid diameter was 7 cm, and nearly half of the treated fibroids were transmural. Reported pregnancy-associated complications included fetal macrosomia, hemolysis with elevated liver enzymes and a low platelet count (HELLP syndrome), premature rupture of membranes with meconium-stained amniotic fluid, breech presentation, and pyelonephritis noted at 16 weeks’ gestation. Since the publication of this pregnancy review, the total number of pregnancies after TFA has risen to 44, resulting in 27 deliveries (11 vaginally, 16 via C/S) [43].
Keltz and colleagues reviewed the literature on pregnancy after hyperthermic ablation of uterine fibroids, including after FUS and RF ablation, and noted 20 pregnancies after RF ablation involving transcervical, transvaginal, and laparoscopic methodology [44]. Pregnancy outcomes after RF ablation were generally favorable, with one delivery after laparoscopic fibroid ablation (LFA) complicated by fibroid expulsion and postpartum hemorrhage. No abnormal placentation or uterine rupture was noted. They also noted two large series of pregnancies after FUS, representing 78 pregnancies in total, along with 24 additional pregnancies cited as case reports. Of note, there were no instances of uterine rupture after FUS, and outcomes were also typically favorable.
Additionally, Polin and Hur performed a systematic review of pregnancies after RF ablation of uterine fibroids [45•]. Ten papers representing 50 pregnancies (40 after LFA, 10 after TFA) were included in the final review. These pregnancies occurred among 923 treated women: 559 with LFA; 364 with TFA. There were 44 full-term deliveries (24 vaginal, 20 via C/S) and 8 spontaneous abortions (12% of pregnancies), one of occurred in the early midtrimester. No instances of uterine rupture, placenta accreta spectrum, or intrauterine growth restriction were noted. The authors concluded “Almost all pregnancies after radiofrequency ablation of fibroids were full-term deliveries with no maternal or neonatal complications. These findings add to the literature that radiofrequency fibroid ablation may offer a safe and effective alternative to existing treatments for women who desire future fertility.”
Across all RF treatment modalities, there have thus been at least 76 published pregnancies (36 pregnancies after TFA and 40 after LFA) [40•, 45•] with generally favorable outcomes. If one also considers the 102 pregnancies after FUS as tallied by Keltz and Chudnoff, the combined published pregnancy experience after hyperthermic (TFA, LFA, and FUS) fibroid ablation represents a minimum of 178 pregnancies [44].
Discussion
The evidence base in support of TFA as a safe, effective, and durable transcervical fibroid treatment option has grown significantly since a prior review from 2017 [15•]. A search of the available literature via PubMed and other sources has revealed 27 papers published since the 2017 review. There have now been 7 additional clinical research trials/studies and subanalyses along with 3 health economic studies. These include multiple prospective controlled trials, an ongoing global clinical registry, and longitudinal data through more than 5 years post-ablation [13••, 14••, 17••, 21••, 23••, 26•, 46•]. Systematic reviews that included, or were exclusive to, TFA have indicated that TFA is safe, effective, and compares favorably against other hyperthermic fibroid ablation methods such as FUS and LFA [32, 33•, 34••]. In total, along with the FAST-EU study, the published data cited herein on TFA represents the treatment of more than 900 fibroids among 463 women.
The 1-, 2-, and 3-year data from the SONATA FDA pivotal clinical trial reinforce the earlier 12-month data from the FAST-EU trial and demonstrate durable clinical improvement over 3 years. It should be mentioned that 65% of women in the SONATA trial reported at least a 50% reduction in menstrual bleeding at 12 months, exceeding the FDA requirement for 50% of patients to have experienced that level of bleeding reduction at 12 months. The results from VITALITY provide additional longitudinal data on TFA demonstrating a low reintervention rate (11.8%) through more than 5 years and are consistent with the 8.2% 3-year cumulative reintervention rate in the SONATA Clinical Trial. While direct, comparative data is not yet available regarding the durability of TFA against that of other fibroid treatments, TFA reintervention rates from 12 to 64 months compare favorably to those of myomectomy, UAE, and other alternatives to hysterectomy [17••, 33•].
The subanalysis from Shifrin et al. of 19 women with fibroids > 5 cm treated in the FAST-EU and SONATA studies, along with the retrospective analysis by Piriyev and colleagues of 50 women with large fibroids, demonstrates the potential applicability of TFA to treat a wide range of fibroid sizes. While operative hysteroscopy, either with resectoscopy or morcellation devices, can address many submucous fibroids, large type 1 and deep/large type 2 fibroids pose challenges, including an increased risk of complications and the need to multistage the procedure [47, 48]. The inability of operative hysteroscopy to treat deeper fibroids that cannot be directly viewed with the endoscope itself, as well as large submucous fibroids, is a limitation of this modality. The integration of intrauterine sonography with RF ablation, as is coupled in the Sonata device, enables transcervical ablation of intramural, transmural, and nonpedunculated subserous fibroids, along with nonpedunculated submucous fibroids. This provides advantages for patient safety and recovery and enables an outpatient site of service for the treatment of nearly all uterine fibroid types.
The OPEN clinical trial found that TFA is associated with a minimal risk of intrauterine adhesiogenesis. The lack of adhesion formation noted by the independent committee in 34 women is encouraging, given that 6 patients in the study had apposing myomata that were ablated. This is in contrast to the significant risk of adhesions after many other transcervical procedures (e.g., hysteroscopic resection of apposing fibroids, endometrial ablation, dilatation, and curettage), which may create challenges for subsequent access to the endometrial cavity [49,50,51,52].
Finally, while safety in women who desire future pregnancy remains a subject of ongoing study, there is reassuring published data involving nearly 80 women in case series involving TFA or LFA and over 100 after FUS. Indeed, FUS is a thermal ablation modality that is considered by the FDA to be compatible with future pregnancy, based on submitted outcomes of 118 pregnancies [15•, 53].
As TFA enables transcervical treatment of fibroids beyond those amenable to hysteroscopic resection or morcellation, it should be reasonably considered as a primary focal treatment option for women as soon as they are diagnosed with symptomatic fibroids. TFA can be used for fibroids up to 8–10 cm in diameter in women with as many as 10 nonpedunculated fibroids. TFA is performed at a significantly lower cost compared to hysterectomy or transperitoneal myomectomy. The procedure is well-tolerated by patients, is performed on an outpatient basis, preserves the uterus, and enables prompt return to normal activity and work. As was noted in 2017, TFA “…could significantly change the current paradigm in which women either lose their uteri or potentially undergo surgical and radiologic procedures that have significant drawbacks in terms of invasiveness and recovery time or require multiple treatment sessions” [15•].
Given a disturbing lack of access to minimally invasive, uterus-conserving fibroid treatments among women of color and other underserved populations, more widespread use of TFA could enable broader availability of outpatient, single-treatment fibroid care delivered by gynecologists without a requirement for general anesthesia or even a hospital operating room [9, 10, 54]. Transcervical fibroid ablation could also potentially address the significant number of unnecessary hysterectomies performed for symptomatic uterine fibroids and avoid the significant morbidities and costs associated with this more radical fibroid treatment option [55]. Finally, there is an unmet need for a less invasive, less morbid approach to the treatment of solitary intramural fibroids (including type 3 myomata) for which hysterectomy or myomectomy is commonly applied. By providing an incisionless, nonresective approach for the treatment of uterine fibroids, this unmet need can be addressed by TFA.
Conclusions
Transcervical fibroid ablation is an outpatient treatment modality that can safely address all nonpedunculated uterine fibroid types and is associated with high patient satisfaction, significant durable symptom relief, and a rapid return to work and normal activity. The growing evidence base confirms TFA as a safe and effective treatment that meets a significant unmet clinical need for fibroid treatments, including among underserved populations.
References
Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743–8. https://doi.org/10.1016/s0029-7844(98)00558-4.
Di Spiezio SA, Mazzon I, Bramante S, et al. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update. 2008;14:101–19. https://doi.org/10.1093/humupd/dmm041.
Luo X, Lim CED, Li L, Wong WSF. Hysteroscopic appearance of endometrial cavity after microwave endometrial ablation. J Minim Invasive Gynecol. 2010;17:30–6. https://doi.org/10.1016/j.jmig.2009.09.012.
MacMahon C, Hatti A, Bakour S, Ewies AAA. Challenges of endometrial assessment after ablation in women with postmenopausal bleeding – a case series. J Obstet Gynaecol. 2018;38:432–4. https://doi.org/10.1080/01443615.2017.1306838.
Wortman M, Daggett A, Deckman A. Ultrasound-guided reoperative hysteroscopy for managing global endometrial ablation failures. J Minim Invasive Gynecol. 2014;21:238–44. https://doi.org/10.1016/j.jmig.2013.09.011.
Ahonkallio SJ, Liakka AK, Martikainen HK, Santala MJ. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69–71. https://doi.org/10.1016/j.ejogrb.2009.06.014.
Endovascular Today. FDA approves InSightec’s Next-Generation ExAblate Fibroid Treatment System. Updated October 6, 2015. Accessed March 2, 2022. https://evtoday.com/news/fda-approves-insightecs-next-generation-exablate-fibroid-treatment-system.
Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210:194–9. https://doi.org/10.1016/j.ajog.2013.08.008.
Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847–55. https://doi.org/10.1016/j.jmig.2018.08.013.
Recent papers of particular interest have been highlighted as: • Of importance •• Of major importance
Altgassen C, Kuss S, Berger U, Löning M, Diedrich K, Schneider A. Complications in laparoscopic myomectomy. Surg Endosc. 2006;20:614–8. https://doi.org/10.1007/s00464-004-2181-8.
Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17:551–4. https://doi.org/10.1016/j.jmig.2010.04.015.
Pistofidis G, Makrakis E, Balinakos P, Dimitriou E, Bardis N, Anaf V. Report of 7 uterine rupture cases after laparoscopic myomectomy: update of the literature. J Minim Invasive Gynecol. 2012;19:762–7. https://doi.org/10.1016/j.jmig.2012.07.003.
Badial G, Fagan PJ, Masood M, Chakravarti S. Spontaneous uterine rupture at 22 weeks’ gestation in a multipara with previous hysteroscopic resection of fibroid. BMJ Case Rep. 2012;2012. https://doi.org/10.1136/bcr.11.2011.5129.
Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473–80. https://doi.org/10.1055/s-0037-1607264.
• Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. https://doi.org/10.1067/mob.2003.99. Provides prevalence data for uterine fibroids among White and Black women.
Dallas K, Dubinskaya A, Andebrhan SB, et al. Racial disparities in outcomes of women undergoing myomectomy. Obstet Gynecol. 2021.
Gartner DR, Delamater PL, Hummer RA, Lund JL, Pence BW, Robinson WR. Integrating surveillance data to estimate race/ethnicity-specific hysterectomy inequalities among reproductive-aged women: who’s at risk. Epidemiology. 2020;31:385–92. https://doi.org/10.1097/EDE.0000000000001171.
Gartner DR, Delamater PL, Hummer RA, Lund JL, Pence BW, Robinson WR. Patterns of black and white hysterectomy incidence among reproductive aged women. Health Serv Res. 2021;00:1–7. https://doi.org/10.1111/1475-6773.13633.
Katon JG, Bossick AS, Doll KM, et al. Contributors to racial disparities in minimally invasive hysterectomy in the US Department of Veterans Affairs. Med Care. 2019;57:930–6. https://doi.org/10.1097/MLR.0000000000001200.
Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81–94. https://doi.org/10.1016/j.ogc.2016.11.007.
• Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012: 194839. https://doi.org/10.1155/2012/194839. Review of radiofrequency ablation and multiple studies related to its applications for uterine fibroids.
•• hudnoff S, Guido R, Roy K, Levine D, Mihalov L, Garza-Leal JG. Ultrasound-guided transcervical ablation of uterine leiomyomas. Obstet Gynecol. 2019;133:13–22. Provides 12-month data from the 147-patient SONATA clinical trial.
•• Brölmann H, Bongers M, Garza-Leal JG, et al. The FAST-EU trial: 12-month clinical outcomes of women after intrauterine sonography-guided transcervical radiofrequency ablation of uterine fibroids. Gynecol Surg. 2016;13:27–35. https://doi.org/10.1007/s10397-015-0915-3. Initial clinical trial of transcervical fibroid ablation.
• Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata System. Curr Obstet Gynecol Rep. 2017;6:67–73. https://doi.org/10.1007/s13669-017-0194-2. The 2017 review of transcervical fibroid ablation.
Bongers M, Brölmann H, Gupta J, Garza-Leal JG, Toub D. Transcervical, intrauterine ultrasound-guided radiofrequency ablation of uterine fibroids with the VizAblate® System: three- and six-month endpoint results from the FAST-EU study. Gynecol Surg. 2015;12:61–70. https://doi.org/10.1007/s10397-014-0873-1.
•• Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19–23. https://doi.org/10.1089/gyn.2018.0051. Long-term data regarding transcervical fibroid ablation, including improvements in quality of life, symptom reduction, and surgical reintervention, with a mean 64-month follow-up.
• Shifrin G, Engelhardt M, Gee P, Pschadka G. Transcervical fibroid ablation with the Sonata™ system for treatment of submucous and large uterine fibroids. Int J Gynaecol Obstet. 2021;155:79–85. https://doi.org/10.1002/ijgo.13638. Subanalysis of data showing transcervical fibroid ablation is effective for myomata > 5 cm, as well as submucous fibroids.
Yoon SW, Cha SH, Ji YG, Kim HC, Lee MH, Cho JH. Magnetic resonance imaging-guided focused ultrasound surgery for symptomatic uterine fibroids: estimation of treatment efficacy using thermal dose calculations. Eur J Obstet Gynecol Reprod Biol. 2013;169:304–8. https://doi.org/10.1016/j.ejogrb.2013.02.023.
Okada A, Morita Y, Fukunishi H, Takeichi K, Murakami T. Non-invasive magnetic resonance-guided focused ultrasound treatment of uterine fibroids in a large Japanese population: impact of the learning curve on patient outcome. Ultrasound Obstet Gynecol. 2009;34:579–83. https://doi.org/10.1002/uog.7454.
•• Lukes A, Green MA. Three-Year Results of the SONATA Pivotal Trial of Transcervical Fibroid Ablation for Symptomatic Uterine Myomata. J Gynecol Surg. 2020;36:228–33. https://doi.org/10.1089/gyn.2020.0021. 36-month results from the SONATA clinical trial.
Yang JH, Chen MJ, Wu MY, Chao KH, Ho HN, Yang YS. Office hysteroscopic early lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil Steril. 2008;89:1254–9. https://doi.org/10.1016/j.fertnstert.2007.05.027.
•• Bongers M, Quinn SD, Mueller MD, et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122–5. https://doi.org/10.1016/j.ejogrb.2019.09.013. Prospective study of second-look hysteroscopy after transcervical fibroid ablation establishing a minimal risk of intrauterine adhesion formation.
Wamsteker K, De Blok SJ. Diagnostic hysteroscopy: technique and documentation. In: Sutton C, Diamon M, editors. Endoscopic surgery for gynecologists: technique and documentation. Lippincott Williams & Wilkins Publishers; 1995. p. 263–76.
• Piriyev E, Schiermeier S, Bends R, Römer T. Transcervical radiofrequency ablation of fibroids that are 5 cm or larger in women with abnormal uterine bleeding. J Gynecol Obstet Hum Reprod. 2022;51: 102303. https://doi.org/10.1016/j.jogoh.2021.102303. Single-center case series (n = 50) evaluating outcomes of TFA in women with large fibroids.
• Christoffel L, Römer T, Schiermeier S. Transcervical radiofrequency ablation of uterine fibroids global registry (SAGE): study protocol and preliminary results. Med Devices (Auckl). 2021;14:77–84. Description and initial safety outcomes of the SAGE clinical registry of transcervical fibroid ablation.
• Huirne J, Brooks E. Improvement in health utility after transcervical radiofrequency ablation of uterine fibroids with the sonata system: health utility after radiofrequency ablation. Eur J Obstet Gynecol Reprod Biol. 2018;224:175–80. https://doi.org/10.1016/j.ejogrb.2018.03.053. A key health economic outcome paper based on data from the FAST-EU clinical trial.
• Roy K, Robinson JK. Durable improvement in generic and fibroid-specific quality of life in women treated with transcervical fibroid ablation with the Sonata System after three years. J Gynecol Surg. 202.1https://doi.org/10.1089/gyn.2021.0073. Subanalysis of the SONATA clinical trial that demonstrates long-term improvements in quality of life after TFA.
• Brooks E, Mihalov L, Delvadia D, et al. The COMPARE study: facility costs associated with hysterectomy, myomectomy, and the Sonata procedure for treatment of uterine fibroids. Managed Care 201940–45. One of three comparative health economic outcome studies regarding TFA.
• Brooks E, Mihalov L, Delvadia D, et al. The INSPIRE comparative cost study: 12-month health economic and clinical outcomes associated with hysterectomy, myomectomy, and treatment with the Sonata System. Clinicoecon Outcomes Res. 2020;12:1–11. https://doi.org/10.2147/CEOR.S214755. One of three comparative health economic outcome studies regarding TFA.
• Brooks EA, Singer AM, Delvadia DR, et al. The CHOICES study: facility level comparative cost, resource utilization, and outcomes analysis of myomectomy compared to transcervical fibroid ablation. Clinicoecon Outcomes Res. 2020;12:299–306. https://doi.org/10.2147/CEOR.S253891. One of three comparative health economic outcome studies regarding TFA.
Taheri M, Galo L, Potts C, Sakhel K, Quinn SD. Nonresective treatments for uterine fibroids: a systematic review of uterine and fibroid volume reductions. Int J Hyperthermia. 2019;36:295–301. https://doi.org/10.1080/02656736.2018.1564843.
• Bradley LD, Pasic RP, Miller LE. Clinical performance of radiofrequency ablation for treatment of uterine fibroids: systematic review and meta-analysis of prospective studies. J Laparoendosc Adv Surg Tech A. 2019;29:1507–17. https://doi.org/10.1089/lap.2019.0550. A systematic review of radiofrequency ablation of uterine fibroids, including TFA.
•• Arnreiter C, Oppelt P. A systematic review of the treatment of uterine myomas using transcervical ultrasound-guided radiofrequency ablation with the Sonata System. J Minim Invasive Gynecol. 2021;28:1462–9. https://doi.org/10.1016/j.jmig.2021.04.009. Systematic review specific to TFA with the Sonata System.
Römer T, Bends R, Christoffel L, et al. Behandlung von symptomatischen Myomen mit der transzervikalen ultraschallgesteuerten Radiofrequenzablation-Indikationen, Durchführung, Ergebnisse und Komplikationen-Expertenkonsensus 2020. Teil 1: Prävalenz, Klassifikation, Diagnostik und etablierte Therapien beim Uterus myomatosus. Frauenarzt. 2021;62:88-94.
Römer T, Bends R, Christoffel L, et al. Behandlung von symptomatischen Myomen mit der transzervikalen ultraschallgesteuerten Radiofrequenzablation-Indikationen, Durchführung, Ergebnisse und Komplikationen-Expertenkonsensus 2020. Teil 2: Die transzervikale Radiofrequenzablation (TRFA) – Methode, Indikationen, Ergebnisse und Vergleich mit anderen Therapien. Frauenarzt. 2021;62:162-168.
• Römer T, Bends R, Christoffel L, et al. The significance of transcervical ultrasound-guided radiofrequency ablation in the treatment of symptomatic fibroids: results of an expert consensus from German-speaking countries. Arch Gynecol Obstet 20221–6. https://doi.org/10.1007/s00404-022-06516-1. A consensus guideline about TFA from German, Swiss, and Austrian gynecologists.
ACOG. Management of symptomatic uterine leiomyomas: ACOG practice bulletin, Number 228. Obstet Gynecol, 2021;137:1131–1133.
Bends R, Toub DB, Römer T. Normal spontaneous vaginal delivery after transcervical radiofrequency ablation of uterine fibroids: a case report. Int J Womens Health. 2018;10:367–9. https://doi.org/10.2147/IJWH.S165959.
• Christoffel L, Bends R, Toub D, et al. Pregnancy outcomes after transcervical radiofrequency ablation of uterine fibroids with the Sonata System. J Gynecol Surg. 2021. https://doi.org/10.1089/gyn.2021.0136. Case series of pregnancy outcomes after TFA.
Garza-Leal JG, León IH, Toub D. Pregnancy after transcervical radiofrequency ablation guided by intrauterine sonography: case report. Gynecol Surg. 2014;11:145–9. https://doi.org/10.1007/s10397-013-0830-4.
Pschadka G, Engelhardt M, Niehoff C, Toub D. Term delivery in an infertile patient after transcervical radiofrequency fibroid ablation and assisted reproductive technology. J Gynecol Surg. 2019;35:253–5. https://doi.org/10.1089/gyn.2019.0001.
Gynesonics (2022). Pregnancy database [Unpublished raw data].
Keltz J, Levie M, Chudnoff S. Pregnancy outcomes after direct uterine myoma thermal ablation: review of the literature. J Minim Invasive Gynecol. 2017;24:538–45. https://doi.org/10.1016/j.jmig.2017.01.009.
• Polin M, Hur HC. Radiofrequency ablation of uterine fibroids and pregnancy outcomes: an updated review of the literature. J Minim Invasive Gynecol 2022S1553–4650(22)00045. https://doi.org/10.1016/j.jmig.2022.01.015. A general review of pregnancy outcomes after ablation of uterine fibroids with radiofrequency energy.
• Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the SONATA pivotal trial. J Gynecol Surg. 2019;35:345–349. https://doi.org/10.1089/gyn.2019.0012. Two-year data from the SONATA clinical trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Hansen Lindner reports personal fees from Gynesonics, outside the submitted work. Dr. Roy reports support from Gynesonics, outside the submitted work. Dr. Toub reports personal fees from Gynesonics, outside the submitted work. In addition, Dr. Toub has a patent US 8,992,427 B2 issued, and a patent US 9,861,336 B2 issued.
Human and Animal Rights and Informed Consent
Informed consent was obtained from all individual participants included in the studies mentioned in this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Uterine Fibroids and Endometrial Lesions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindner, L.H., Roy, K. & Toub, D.B. Transcervical Fibroid Ablation (TFA) with the Sonata System: Updated Review of a New Paradigm for Myoma Treatment. Curr Obstet Gynecol Rep 11, 238–248 (2022). https://doi.org/10.1007/s13669-022-00341-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-022-00341-8