Abstract
Objective
Stereotactic body radiation therapy (SBRT) is an attractive option for prostate cancer due to its short treatment duration and cost. In this report, we compare the efficacy and toxicity outcomes of prostate cancer patients treated with SBRT to those who received intensity-modulated radiation therapy (IMRT).
Methods
Two hundred sixty-three patients with localized prostate adenocarcinoma were included, ranging from clinically very low- to high-risk groups. We retrospectively compare consecutive patients treated with SBRT with consecutive patients treated with conventionally fractionated IMRT. For most patients, SBRT was delivered to a total dose of 36.25 Gy in five fractions and IMRT to 75.6 Gy in 42 fractions. To minimize selection bias, we perform propensity score analyses.
Results
The treatment groups became similar after propensity matching with absolute standard bias reduced to ≤0.19. For the first analysis, 5-year actuarial survival was 90.8 and 88.1 % in SBRT and IMRT groups, respectively (p = 0.7260), while FFBF was 88.7 and 95.5 %, respectively (p = 0.1720). For the second analysis (accounting for risk group), actuarial 5-year survival was 96.7 and 87.1 % in the SBRT and IMRT groups, respectively (p = 0.3025), while FFBF was 89.7 and 90.3 %, respectively (p = 0.6446). Toxicity did not exceed grade 3 in any of the studied patients. The highest recorded genitourinary toxicity at the time of latest follow-up was grade 2.
Conclusion
Our data support the hypothesis that SBRT has non-inferior efficacy and toxicity rates as IMRT. Given the lower cost and convenience for patients, SBRT may be considered as an alternative treatment for localized prostate cancer.
Similar content being viewed by others
Introduction
Intensity-modulated radiation therapy (IMRT) is one of the standard radiotherapeutic techniques for the definitive treatment of localized prostate cancer. There are several decades of data supporting excellent biochemical control and overall survival for delivery of conventionally fractionated radiation therapy to the prostate and surrounding tissue at risk, while maintaining acceptable toxicity [1]. IMRT was adopted prior to long-term data from phase 3 trials and demonstrated a superior therapeutic ratio [2–4]. The rationale was based on IMRT being a technical innovation on an already proven treatment modality, conventionally fractionated radiation therapy, delivered by three-dimensional conformal radiation therapy (3DCRT) or two-dimensional conventional techniques (2DCT) with sufficient long-term data to support its use as standard of care.
Stereotactic body radiation therapy (SBRT) is a technologically advanced treatment modality that takes advantage of the low alpha-beta ratio of prostate cancer in order to deliver extreme hypofractionated radiation therapy. As a result, SBRT achieves high biological equivalent doses substantially above those attempted in IMRT.
There has been further interest in SBRT for prostate cancer due to its convenience for patients (duration of 1 to 2 weeks, as compared to 8 to 9 weeks of IMRT) and lower cost [5]. SBRT has been considered a different treatment modality than conventionally fractionated therapy and IMRT due to the complex radiobiological differences of extreme hypofractionation compared to standard fractionation. Late responding normal tissue surrounding the malignant tissue may be more susceptible to late toxicity after receiving high doses delivered in fewer fractions. Therefore, one may argue that extreme hypofractionated schemes require many years of follow-up to accurately assess late toxicity.
It is ideal to adopt a new therapeutic modality that is cheaper and more convenient for patients once randomized trials demonstrate non-inferior outcomes and acceptable toxicity. However, by the time phase 3 trial data mature in localized prostate cancer, we may be faced with new scientific and bureaucratic pressures that push prostate cancer treatment in a different direction. Reliance on randomized trials alone is not practical, given the past difficulty in enrolling patients into phase 3 trials that compare prostate cancer treatment modalities head-to-head. The logic used to adopt IMRT as standard of care by means of long-term data from 3DCRT/2DCT can be similarly applied to SBRT, by using data from high-dose rate (HDR) monotherapy as a surrogate, in which data supporting the latter [6–11] justify use of the former.
Therefore, a balance is required which allows new technologies to be offered to patients prior to maturation of long-term data from phase 3 trials. This can be achieved for prostate SBRT through evidenced-based treatment protocols based on long-term data from HDR monotherapy and 5-year data from phase 2 trials of SBRT. Irrespective of the controversy surrounding SBRT, there have been early publications which report promising clinical efficacy of SBRT for localized prostate cancer, along with low rates of toxicity [12]. However, the literature is limited by short-/intermediate-term follow-up. As we await long-term data from ongoing randomized studies, there has been a high level of interest to compare extreme hypofractionation to conventionally fractionated approaches.
We seek to enhance the perspective on this topic by comparing our experience with SBRT and IMRT. Yu et al. published an analysis of Medicare beneficiaries comparing toxicity and cost between SBRT and IMRT.
At 2-year follow-up, they demonstrated a small but statistically significant increase in toxicity in patients who received SBRT as compared to IMRT, while the former had a substantially lower financial cost [5]. Our current study similarly compares SBRT toxicity to IMRT, but additionally analyzes treatment outcome by means of biochemical control and overall survival utilizing propensity score modeling.
Methods
Stereotactic body radiation therapy
All 142 consecutive men with localized prostate cancer treated between 2007 through 2012 at our satellite radiation oncology center with SBRT as monotherapy were included in this IRB-approved study. The CyberKnife system with multi-plan inverse treatment planning and motion tracking of internal fiducials was used. Treatment planning began with fiducial placement into the prostate. A CT scan was obtained 10–14 days later. T2 fast echo MRI was obtained and three-dimensionally registered by fiducials to the CT in the majority of patients.
The clinical target volume (CTV) was the prostate for low-risk patients and the prostate plus proximal seminal vesicle base for most intermediate- and high-risk patients. Pelvic lymph nodes were never targeted with SBRT. Five fractions were prescribed to the planning target volume (PTV) that consisted of the CTV with a 5-mm margin in all directions except 3 mm posteriorly. The dose delivered changed over the time of the study. Initially, patients received 35 Gy, followed by 37.5 Gy, and at the time of this publication to 36.25 Gy, driven by data available at that time and standard protocol established by Accuray. At least 95 % of the PTV received the prescribed dose normalized to the 75–85 % isodose line (dose heterogeneity 17–33 %). Less than 1 cm3 of rectum received 36 Gy, 50 % of the prescribed dose could not cross the posterior rectal wall, and <10 cm3 of bladder received 37 Gy. The average CTV and PTV were 56.9 cm3 (std dev 27.7 cm3) and 98.0 cm3 (std dev 48.9 cm3), respectively. Orthogonal 120-kV x-ray image pairs were obtained throughout treatment for use in motion tracking. Real-time prostate position was locked on by the relative fiducial position on the x-rays. For those patients with evenly distributed fiducials in the prostate quadrants, the prostate’s rotation was also tracked and corrections were made in real time.
Intensity-modulated radiation therapy
All 121 consecutive men with localized prostate cancer between 2007 through 2012 at our hospital radiation oncology center treated with IMRT were included in this IRB-approved study. Of note, IMRT and SBRT are delivered at separate geographic sites within the same radiation oncology department/hospital. The CTV was defined as the prostate with 8-mm margin in all directions except 5 mm posteriorly in low-risk patients, prostate plus seminal vesicles with 5- to 8-mm margin for intermediate-risk patients, and prostate plus seminal vesicles plus true pelvic lymph nodes with 5- to 8-mm margin for high-risk patients. Dose constraints to the rectum were defined as V65 <17 % and V40 <35 %, while the bladder constraint was V65 <25 % and V40 <50 %. All patients were simulated in the supine position with immobilization, full bladder, and empty rectum. CT and MRI treatment planning was completed with merging of the images for contouring of the prostate. Image guidance was provided by BAT ultrasound pretreatment daily and patients were treated with full bladder daily. Usually, five to seven isocentric beams were utilized to treat the prostate with 6 MV photons optimized with an inverse optimization algorithm with at least 95 % of the prostate receiving the prescribed dose. The majority of patients received at least 75.6 Gy to the prostate in 1.8 Gy per fraction. Of those that received less than 75.6 Gy, the majority were stratified into either the very-low-risk or low-risk groups.
Analysis
PSA nadir to date is defined as the lowest PSA value following radiotherapy. Benign PSA bounce was defined as a PSA rise of ≥0.2 ng/mL above its previous nadir with subsequent decline to that nadir or lower. Biochemical failure (BF) was assessed using the nadir +2 (Phoenix) definition. Toxicity was assessed using the Radiation Therapy Oncology Group (RTOG) criteria; acute toxicity occurred within 3 months and late toxicity >3 months following treatment. Risk group was based on the National Cancer Center Network (NCCN) version 2.2014 classification: very low, low, favorable intermediate (one intermediate risk factor), unfavorable intermediate (more than one intermediate risk factor), and high. Since there were few very high risk patients, they were included in the high-risk group.
Statistics
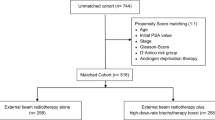
The overall sample is described using measures of central tendency (mean and median) and variation [standard deviation and interquartile range (IQR)], and compared by treatment group using two-sample t tests and Fisher exact tests, as appropriate. To minimize selection bias inherent in treatment group allocation, propensity score modeling was used to match the two groups using a logistic regression approach [13]. To evaluate the robustness of the choice of matching covariates, two sets of characteristics were used for the propensity score modeling: (1) individual patient and tumor characteristics including age, tumor stage, Gleason score, pretreatment PSA, treatment year, and androgen deprivation therapy (ADT) use; and (2) NCCN version 2.2014 risk group, age, treatment year, and ADT use. For each set of matching covariates, various propensity score modeling strategies were employed, including simple 1:1, 1:1 with replacement, 1:1 with caliper, 1:1 optimal, full matching, and sub-classification. The methods were compared in terms of bias reduction using box plots (Fig. 1) and overlap of the propensity scores using box plots. An absolute standard bias measure <0.20 is considered small, and sufficient overlap is required for the propensity scores [14]. As seen in Fig. 1, the method demonstrating optimal bias reduction was 1:1 with caliper for both sets of matching covariates, and was thus chosen as the basis for outcome analysis. Figure 2 demonstrates that bias was reduced to <0.20 for all variables used in both of the matching analyses. Kaplan-Meier estimates of overall survival and freedom from biochemical failure were used to describe the patients overall, and comparisons were accomplished using log-rank statistics [15, 16].
Results
Patient and treatment characteristics
The outcomes of 263 patients were analyzed, with median follow-up of 43 months (IQR 24–58). The SBRT treatment group of 142 consecutively treated patients had median follow-up of 34 months (IQR 22–54). The IMRT treatment group of 121 consecutively treated patients had median follow-up of 51 months (IQR 33–64). SBRT doses were 35.0 Gy in five fractions (4 %), 36.25 Gy in five fractions (75 %), and 37.5 Gy in five fractions (21 %) of patients. IMRT was delivered in conventional fractionation with 1.8–2.0 Gy per fraction, median dose 75.6 Gy delivered in 42 fractions, specifically, 6 % 79.2 Gy, 18 % 78 Gy, 59 % 75.6 Gy, and 17 % 72 Gy.
The SBRT group comprised patients with younger age and lower risk disease determined by the pooled results of a two-sample t test and Fisher’s exact test, respectively, as compared to the IMRT group (Table 1).
Patients treated with IMRT were more than twice as likely to receive ADT compared with SBRT, which was consistent for each risk group.
Unmatched outcome
At most recent follow-up, there were no deaths from prostate cancer in either treatment group. All patients died of causes unrelated to malignancy in the SBRT group, whereas 9 of 15 patients in the IMRT group died of cancer-related causes not associated with prostate adenocarcinoma—primaries of the lung, pancreas, esophagus, and leukemia. Metastatic progression occurred after biochemical failure in three of six patients in the SBRT group and three of nine patients in the IMRT group.
Treatment with both modalities was well tolerated. Acute and late genitourinary (GU) toxicity experienced after treatment did not exceed grade 3. The most severe acute and late gastrointestinal (GI) toxicity was grade 2. All grade 3 toxicities subsided at most recent follow-up. Grade 2 GU toxicity persisted in 12 and 14 % of patients in the IMRT and SBRT groups, respectively. Grade 2 GI toxicity persisted in 1 and 3 % of IMRT and SBRT groups, respectively.
Grade 3 erectile dysfunction (ED) was defined as inability to achieve an erection sufficient for intercourse despite the use of medications or the need for penile prosthesis. The analysis of this particular outcome excluded patients on long-term ADT and those who had grade 3 ED at baseline. At most recent follow-up, grade 3 ED persisted in 17 % of those who received IMRT and in 6 % of those who received SBRT.
Matched analyses
In order to minimize selection bias resulting from confounding patient and treatment differences between the SBRT and IMRT treatment groups, we performed two additional propensity score analyses which matched patients between the two groups. The first analysis relied on 1:1 propensity score matching with a caliper using age, Gleason score, pretreatment PSA, treatment year, tumor stage, and ADT use. The resulting treatment groups became similar, with the absolute standard bias reduced to ≤0.19 for all covariates (Table 2). For this sample, the 5-year actuarial survival (Table 3 and Fig. 3) was 90.8 and 88.1 % in the SBRT and IMRT treatment groups, respectively (p = 0.7260, overall comparison between curves). For the same sample, the 5-year actuarial FFBF was 88.7 and 95.5 % in the SBRT and IMRT treatment groups, respectively (p = 0.1720 overall, Table 3 and Fig. 3).
Similarly, we generated a propensity score analysis using 1:1 matching with a caliper to account for differences in NCCN version 2.2014 risk group, ADT use, age, and treatment year. As a result of the matching, the resulting treatment groups again became similar, for which the absolute standard bias was reduced to ≤0.19 for all risk groups (Table 2). For this sample, the 5-year actuarial of overall survival (Table 3 and Fig. 3) was 96.7 and 87.1 % in the SBRT and IMRT treatment groups, respectively (p = 0.3025, overall comparison between curves). For the same sample, the 5-year actuarial of FFBF was 89.7 and 90.3 % in the SBRT and IMRT treatment groups, respectively (p = 0.6446 overall, Table 3 and Fig. 3).
Discussion
Given the retrospective observational nature of our study, two separate matching methods were used to reduce bias introduced by confounding prognostic factors with treatment, and thus balance differences between treatment groups. Results from 1:1 propensity score matching with caliper based on age, Gleason score, pretreatment PSA, treatment year, tumor stage, and ADT use demonstrate no statistically significant differences in outcomes between SBRT and IMRT treatment groups. Consistent with these findings, similar matching based on NCCN risk group, ADT use, age, and treatment year also demonstrate no statistically significant differences in outcomes between treatment groups. Ideally, propensity score matching seeks to minimize differences between imbalanced groups in order to create samples similar to those in a prospective randomized study. Thus, separate analyses produced similar results, in which there was no difference in overall survival or FFBF in our patient population.
A common critique of SBRT for prostate cancer is that adopting it as routine practice should await long-term results from randomized phase 3 trials. It has been suggested that an ongoing Swedish phase 3 trial of hypofractionated radiotherapy delivered in >5 fractions (ISRCTN45905321) [17] will help shed light on this topic once published [18]. Several phase 3 trials of hypofractionation in >5 fractions have been reported, albeit using moderate hypofractionation, of which four of five show no significant difference in rates of late toxicity [19–23]. The trial with the longest follow-up (90 months) demonstrated that a conventionally fractionated scheme was an independent prognostic factor for worse late GU toxicity [21].
Several prospective studies of SBRT have reported high rates of biochemical control with acceptable toxicity. King et al. demonstrate the favorable therapeutic ratio in a consortium of patients from phase 2 trials who were treated between 2003 and 2011 at eight institutions. Five-year bRFS was achieved in 95, 84, and 81 % of low-, intermediate-, and high-risk patients, respectively. The use of ADT and SBRT dose did not significantly affect bRFS even after stratifying by risk group [12]. In a separate publication, King et al. describe patient-reported quality of life (QOL) from a sample of 864 patients from four phase 2 studies that evaluate SBRT. They demonstrated urinary and bowel QOL decline within the first 3 months after treatment, recovering by 6 months, remaining stable at 3 years’ follow-up, and subsequently achieving superior QOL as compared to baseline [24]. This trend was independent of severity of acute toxicity and duration of ADT use. Katz et al. compared similar prospective QOL measures in patients who received SBRT and radical prostatectomy. Both modalities resulted in decline of urinary and bowel QOL at 1 month, with an upward trend at later time points. After SBRT, QOL recovered by 6 months to levels not significantly different than baseline (latest 36 months), whereas QOL after surgery remained significantly lower compared to baseline at all time points [25]. Katz et al. series of 477 patients with low- and intermediate-risk prostate cancer treated with SBRT demonstrated a 7-year actuarial FFBF of 95.6 and 89.6 % for low- and intermediate-risk patients, respectively. No late grade 3 GI toxicity was noted and late GU toxicity was limited to 1.7 % [26].
Comparison of SBRT to IMRT for localized prostate cancer has been published in one other study to date [5]. An analysis of Medicare beneficiaries in the United States (age >65) treated from 2008 through 2011 showed greater toxicity associated with SBRT than with IMRT. The absolute difference in toxicity at the latest time point of 24 months was 8 % (p = 0.01). However, toxicity was neither graded nor reported subjectively. The radiation treatment platform used for SBRT was not reported nor was there a dose/volume analysis presented. It was reported based on claims submitted via international coding of disease (ICD-9). Differences in toxicity may have little clinical relevance if, for example, an 8 % difference comprised mostly of grade 1 or 2 toxicity. Furthermore, late toxicity will be inherently missed in a study which does not exceed 24 months’ follow-up. The Medicare analysis does not report PSA outcome or survival, as this information is not obtainable through the Medicare database. Nonetheless, Yu et al. provide an important perspective on two distinct treatment modalities. The financial cost was reported to be substantially lower for SBRT than IMRT, with mean cost for a full course of IMRT $7348 more than SBRT.
Our current series complements the Medicare analysis by Yu et al. Together, our publications enable a hypothesis to be generated for future prospective trials, in which SBRT may be non-inferior to IMRT in regard to GU/GI toxicity, survival, and biochemical outcome, while having a substantially lower financial cost. Limitations of our study include the selection bias that arises in retrospective observational series. However, we attempt to minimize such bias by incorporating two statistical propensity-matched analyses.
References
Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H (2006) Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol 176:1415–1419
Sheets NC, Goldin GH, Meyer AM, et al. (2012) Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. Jama 307:1611–1620
Goodman A (2012) Prostate cancer studies compare outcome, toxicities, and cost. The ASCO Post 2012;3:5:http://www.ascopost.com/issues/march-15-2012/prostate-cancer-studies-compareoutcomes,-toxicities,-and-costs.aspx. Accessed 26 August 2014
Nguyen PL, Gu X, Lipsitz SR, et al. (2011) Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol 29:1517–1524
Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, Gross CP (2014) Stereotactic body radiation therapy versus intensity modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol 32:1195–1201
Barkati M, Williams SG, Foroudi F, et al. (2012) High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: a phase II trial. Int J Radiat Oncol Biol Phys 82:1889–1896
Demanes DJ, Martinez AA, Ghilezan M, et al. (2011) High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 81:1286–1292
Corner C, Rojas AM, Bryant L, Ostler P, Hoskin P (2008) A phase II study of high-dose-rate after loading brachytherapy as monotherapy for treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys 72:441–446
Hoskin P, Rojas A, Lowe G, et al. (2012) High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int J Radiat Oncol Biol Phys 82:1376–1384
Yoshioka Y, Konishi K, Sumida I, et al. (2011) Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys 80:469–475
Tselis N, Tunn UW, Chatzikonstantinou G, et al. (2013) High dose rate brachytherapy as monotherapy for localised prostate cancer: a hypofractionated two-implant approach in 351 consecutive patients. Radiat Oncol 8:115
King CR, Freeman D, Kaplan I, et al. (2013) Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 109:217–221
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 58:457–481
Kleinbaum DG, Klein M (2005) Survival analysis: a self-learning text, 3rd edn. NY, New York
Widmark A (2009) ISRCTN: Phase III study of hypofractionated radiotherapy of intermediate risk localized prostate cancer. 2009. http://www.controlled-trials.com/ISRCTN45905321
D’Amico AV (2014) Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: less cost at the expense of more genitourinary toxicity is a concerning but testable hypothesis. J Clin Oncol 32:1183–1185
Lukka H, Hayter C, Julian JA (2005) Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 23:6132–6138
Pollack A, Walker G, Horwitz EM, et al. (2013) Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 31:3860–3868
Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J (2011) Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys 81:1271–1278
Dearnaley D, Syndikus I, Sumo G, et al. (2012) Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomized controlled trial. Lancet Oncol 13:43–54
Arcangeli G, Fowler J, Gomellini S, et al. (2011) Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 79:1013–1021
King CR, Collins S, Fuller D, et al. (2013) Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 87:939–945
Katz A, Ferrer M, Suárez JF (2012) Multicentric Spanish group of clinically localized prostate cancer. Comparison of quality of life after stereotactic body radiotherapy and surgery for early stage prostate cancer. Radiat Oncol 7:194–202
Katz A, Dong J (2014) Stereotactic body radiotherapy as treatment for organ confined low and intermediate risk prostate carcinoma, a 7-year study. Front Oncol 4:240
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest statement
The authors Dr. Rachelle Lanciano, Dr. John Lamond, Dr. Steven Arrigo, and Dr. Luther Brady each own a small percentage of the single unit Cyberknife used at the Philadelphia Cyberknife Center. The authors Dr. Caspian Oliai, Matthew Bernetich, Dr. Jun Yang, Dr. Alexandra Hanlon, Michael Good, Dr. Michael Mooreville, and Dr. Bruce Garber do not have any conflicts of interest to report.
Ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The authors Dr. Rachelle Lanciano, Dr. John Lamond, Dr. Steven Arrigo, Dr. Luther Brady, Dr. Caspian Oliai, Matthew Bernetich, Dr. Jun Yang, Dr. Alexandra Hanlon, Michael Good, Dr. Michael Mooreville, and Dr. Bruce Garber all attest that this article does not contain any studies with human or animal subjects performed by any of the authors.
Funding sources
The authors Dr. Rachelle Lanciano, Dr. John Lamond, Dr. Steven Arrigo, Dr. Luther Brady, Dr. Caspian Oliai, Matthew Bernetich, Dr. Jun Yang, Dr. Alexandra Hanlon, Michael Good, Dr. Michael Mooreville, and Dr. Bruce Garber all attest that no funding sources are associated with this article.
Additional information
Our data support the hypothesis that stereotactic body radiation therapy is non-inferior to intensity-modulated radiation therapy for the primary treatment of localized prostate cancer. Given the lower cost and convenience for patients, stereotactic body radiation therapy could be considered for localized prostate cancer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oliai, C., Bernetich, M., Brady, L. et al. Propensity score matched comparison of SBRT versus IMRT for the treatment of localized prostate cancer. J Radiat Oncol 5, 187–195 (2016). https://doi.org/10.1007/s13566-015-0237-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-015-0237-0