Abstract
The research aimed to examine the enhancement effect of Spirulina platensis (blue-green microalga or Cyanobacterium) and Chlorella vulgaris (green microalga) water extracts as biological stimulant in improving growth parameters, chlorophyll content, yield, and fruit quality of tomato plants under salinity stress. Tomato seeds (Solanum lycopersicum L. of cv. Agyad) were soaked in three microalgae water extracts at a 10% concentration (Chlorella vulgaris, Spirulina platensis, and Chlorella:Spirulina at a ratio of 1:1). Chemical and biochemical analyses for the used materials and products were achieved. After germination, tomato transplants were irrigated with saline water at three levels of saline water (2, 4, and 7 dS/m) using sea salt. Successive grown tomato transplant at 7.0 dS/cm were infused in clayey soil. The grown plants were preyed by algal extract and irrigated by the same solution. Vegetative growth, yield, shelf life, and chlorophylls (a and b) were determined. It was found that the highest vegetative growth, yield, and chlorophyll content were measured in tomato transplants with the Spirulina:Chlorella mixture, followed by Spirulina platensis and Chlorella vulgaris water extracts; all of these measurements increased significantly in response to microalgae treatments. Experimental tomato fruits of Chlorella and mixture treatments can stay for 45 days at room temperature. Both algal extracts and their mixture enhanced the bioaccumulation of micronutrients (Fe, Zn, Mn, and Cu), compared with the control, while Chlorella extract surpassed Spirulina and mixture extracts. Concerning the used dried algae and their water extract, Spirulina platensis surpasses Chlorella vulgaris for protein and ash content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Arid and semiarid regions severely suffer from a series of environmental and physical injuries, mainly salinity and drought. The climate in Egypt is characterized by its aridity with high annual potential evapotranspiration. The fertile region in Egypt (East of Nile Delta) was characterized by 15% slightly saline, 13% moderately saline, 2% strongly saline, and 10% for extremely saline level (Hammam and Mohamed, 2020) [1]. It is appearing that there is a strong correlation between the salinity of newly reclaimed land and the amount of groundwater used for irrigation. Around 30–40% of the land in the Nile Delta is salt affected and soils with moderate salinity levels (4–8 dS/m) have the best potential for the implementation of salt tolerant crops. Overcoming the salinity adverse effect, however, appears to be a fast solution and is expected to increase soil salinity. Thus, the use of non-traditional methods, in turn, led to an increase in solute penetration within plant cells as well as hiding the salinity adverse to plant growth. In this action, using the algal extract with special care and attention to freshwater was applied early [2]. Here, the employment of subsurface drainage system controlled on 90% of irrigated lands, however, increases the coast and lakes, soil salinity due to the effect of the shallow saline groundwater and seepage of brackish water from the sea and lakes through more permeable soils [3]. Another way to overcome salinity effect [4] is to produce the halophyte plants, where some of these plants (Suaeda salsa) showed a proper growth under high salinity levels (40 g/l). This technique seems to be on the expense of edible and strategy crops and might increase soil salinity under high saline water when other physical methods like magnetic water was employed; however, [5] showed its enhancing effect on soil moisture, plant growth, yield, and quality. Other traditional methods are increasing of organic manure or biological compounds from algae [6] and bacteria [7].
Tomato is considered an essential source of national agricultural income and occupies a crucial position in exports to the Egyptian economy. Tomato production accounts for approximately 5.3% of the export of Egyptian vegetables, accounting for approximately 555.52 thousand tons worth approximately 87.79 million pounds, and the cultivated area is approximately 490.26 thousand feddans (acre = 2.5 feddan, approximately), with productivity reaching production of approximately 15.7 tons/feddan [8].
Microalgae are a wide-ranging group of prokaryotic and eukaryotic photosynthetic organisms that are able to grow rapidly in both marine and freshwater environments owing to their unicellular structure [9]. Microalgae are mainly classified according to their pigmentation, life cycle, and cell structure, and approximately 800,000 microalgal species exist, of which approximately 50,000 species have been identified [10]. Concerning crop production, microalgae constitute elevated levels of macro- and micronutrients necessary for ideal crop growth and development [11]. Microalgae are used as biostimulators and biofertilizers for agricultural purposes and are considered a cost-effective and environmentally friendly substitute for other products, such as chemical fertilizers and plant growth regulators [12]. Biostimulants act on plant physiology through different pathways, improving crop growth, yield, quality, uptake of nutrients, tolerance to abiotic stresses, and shelf life of the harvest [13,14,15]. Biofertilizers are natural substances that can improve the chemical and biological properties of soil, enhance plant growth, and renovate soil fertility [16]. Several studies have been performed on using algal extracts in the alleviation of abiotic stresses, in addition to their uses in plant nutrition and growth enhancement [2, 6].
Among these, Spirulina spp. and Chlorella spp. are the main microalgal species cultivated and used worldwide [17]. Moreover, algal varieties tested as biofertilizers primarily belong to groups of blue-green algae (Cyanophyta) and green algae (Chlorophyta), which play a crucial role in sustainable agriculture [18, 19]. Among Cyanophyta species, Spirulina platensis can be used as a rich source of macro- and micronutrients for plants [19, 20]. In addition, many studies have shown that green algae can add organic matter, synthesize, and liberate amino acids, vitamins, and auxins, reduce the oxidizable matter content of the soil, provide oxygen to the submerged rhizosphere, improve salinity, and buffer the pH, solubilize phosphate, and increase the fertilizer use efficiency of crop plants [21, 22].
The reduction observed in crop growth is mainly related to the osmotic potential of the root-zone soil solution, which in turn affects seed germination and transplant progress [23]. On the other hand, with the world population increase, food, freshwater, and fuel use increase every day, and a new agriculture type that is sustainable, environmentally friendly, and appropriate for soils needs to be developed and practiced. An innovative strategy is in an approach known as saline agriculture, which is not new and has already been suggested in the last three decades, and [24] concentrated research efforts on the salt tolerance. This has led to plants developing crop production [25]. Numerous research studies have proven the advantageous effect of microalgae in stimulating the growth of plants under stressful conditions such as drought and salt stresses due to their biostimulating and biofertilizing properties, which are beneficial in increasing agricultural sustainability [26]. Many crops, including tomato, are subjected to cell damage due to high salinity and can continue only with reduced yields. In addition, the overuse of chemical fertilizers raises the production costs to the level that the crop cannot be produced and sold in a profitable manner [8].
Growth experiment with tomatoes proved that microalgae fertilization increased tomato plants’ chlorophyll content by 12%, compared to control plants without fertilizers [27]. Using algae extracts led to overcoming the adverse effects of salinity by significantly increasing the yield of plants, especially with Spirulina, which could be attributed to the occurrence of micro- and macronutrients, growth hormones, vitamins, amino acids, and other ingredients in algae that might be responsible for better enhancement in yield production [2, 28].
Previous researches were focused in many objectives mainly aiming at the increasing of plant resistance against biotic and abiotic stresses including salinity drought, frosts, biocontrol, and/or improving the nutritional status. Of these, [2, 29] tomato plants were grown in hydropnic system till 6000 ppm salinity and sprayed with Spirulina extract [2]. Extract was prepared, as described by [30]. Otherwise, [29] used the extract of Chlorella vulgaris for tomato treatment. Culture suspension (CS) was centrifuged; the algae pellet was re-suspended in water (CCS); and this was applied weekly to soil, while algae extract (cell disrupted algae suspension — CDS) was sprayed on leaves bi-weekly. The flowering process, plant morphology, fruit features, and pigment contents were analyzed. In the second set of experiments, the culture suspension per se (CS) was applied to the soil weekly, and CDS was sprayed on leaves bi-weekly. Moreover, [9] used microalgae polysaccharides which represent a potential bioresource for the enhancement and the protection of agricultural crops. We investigate the possibility to use microalgae polysaccharides as a plant biostimulant. The crude polysaccharide extract (PS) from three microalgae strains were applied to Solanum lycopersicum plants by irrigation and compared basing on their effects on shoot and root length, node number, and shoot and root dry weight. The application of 1mg/ml 1 PS from Arthrospira platensis, Dunaliella salina salina, and Porphorydium sp. on tomato plants improved significantly the node number (NN), shoot dry weight (SDW), and shoot length (SS) by 75, 46.6, and 25,26% compared to control, respectively. The current research aimed to examine the enhancement effect of Spirulina platensis (blue-green microalga or Cyanobacterium) and Chlorella vulgaris (green microalga) water extracts as biostimulator in improving the growth parameters, chlorophyll content, yield, and fruit quality of tomato plants under salinity stress.
2 Methods
2.1 Microalgae and their extracts
Two microalgae, Chlorella vulgaris belonging to Chlorophyta and Spirulina platensis belonging to Cyanophyta, used in the current research were massively produced outdoors (Algal Biotechnology Unit, National Research Center, Egypt) via different growth units [31]. The strains’ origin of the two microalgae, culture collection, and strain number are, first, Spirulina platensis (Cyanophyta) was isolated from the brackish Egyptian water in Wady El-Natron district, El-Behara Governorate (30.58333°N 30.33333°E); and the second was Chlorella vulgaris (Sequence ID: LC333291). Both of them were early isolated from the Egyptian environment. More than one mass production systems are routinely performed at Algal Biotechnology unit, NRC. Actually, open ponds (continuous cultivation system 75 m3) are the most proper one. A commercial growth medium was made based on the recommended growth media of [32] for Spirulina platensis and [33] for Chlorella vulgaris. Modification was done to substitute sodium nitrate by the same equivalent content of nitrogen from urea (i.e.; 0.89 and 0.53 g·l/1, respectively).
Outdoor-grown algae (Chlorella vulgaris and Spirulina platensis) were harvested, washed with tap water, dewatered under vacuum filtration, dried at 45 °C using a circulated oven, and sieved for fine powder. Biochemical analysis was performed based on the methods described by [34]. Ashing was performed in a muffle furnace (500 °C), and the residue was dissolved in 2.0 N HCl, quantitively transferred (50 ml), filtered using ashless filter paper, and then subjected to mineral analysis by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Nitrogen (T. N%) was determined [35], in which protein was calculated as T. Nx6.25. Ether extract represented the oil content, which was determined using Soxhelt extract and weight differences. Total carbohydrates were spectrophotometrically determined by the phenol sulfuric method (DuBois et al., 1956). Chlorophyll was extracted by an acetone–hexane mixture (2:3), and absorptions were measured at 645 and 663 nm (Ch a) [36].
For algae extract preparation, two different algal extracts were prepared. The first was prepared from dry algal biomass [2] by soaking it into hot water (10:100 w:v) for 18 h (approximately 1 working day). The fine obtained solution was then used for seed soaking. The second extract was made from wet harvested algal biomass which contains 85–90% of moisture and which in turn seems as a dough. So, we simulate the original form in which algae became saturated by water, [30]. The locally produced algae Chlorella vulgaris and Spirulina platensis (National Research Centre, Cairo, Egypt) were used in the current work through the outdoor mass production (75 m3 capacity), as previously described by [31] using the commercial fertilizer compounds for Chlorella mass production. In the case of Spirulina alga, growth medium was enriched by 16.8 g/l of NaHCO3 [32]. Both of them were harvested by continuous centrifuge (Westvalia Separator, Germany) at 10 m3/h, washed by fresh water, and dried at 55 °C. The water volume:water ratio was 10 g algae:100 ml hot water.
The used biomass was early considered as a natural, rich, and cheaper source of natural constituents mainly amino acids and phytohormones. It is worthily mentioned that a lot of different algal species were used in this topic by many authors. Fresh algal cultures were harvested by continuous centrifugation, dried at 45 °C, and finely ground.
Fine powder was soaked in hot water (70 °C) overnight. Then, the seeds were soaked for 18 h, homogenized by an electromechanical mortar, and recentrifuged. The clear layer was then separated and used (aqueous solution). A readymade algal biostimulator was used, algal biostimulator made from fresh algal biomass was used by freezing it and subjected it after melting to laboratory centrifugation (4000 rpm/5 min). The product was kept on the frigider till its use.
As mentioned above, 18-h soaking time approximately revealed overnight related to working day (2.0 pm–10 am). It was observed that long-time soaking led to algal fermentation due to infection by other microorganisms or self-fermentation.
2.2 Experimental design
First, tomato seeds (Solanum lycopersicum L.) of cv. Agyad 7 purchased from the Horticultural Research Institute, Agriculture Research Center, Ministry of Agriculture, Giza, Egypt, were soaked in algal water extracts for 18 h; this included three microalgae water extracts of 10% concentration (Chlorella vulgaris, Spirulina platensis, and Chlorella:Spirulina in a ratio of 1:1), which is made by soaking dry algal powder in distilled water 1:10 w:v) overnight and then recentrifuged; while control seeds were soaked in freshwater only for the same time. After the soaking period, seeds were dried on filter papers and then sown in 30-cm diameter pots filled with a soil mixture consisting of peat moss with sand at a ratio of 1:2. All pots were irrigated with fresh water until reaching the seedling stage, after which the pots were irrigated with three levels of saline water (2, 4, and 7 dS·m/1) by using sea salt to prepare saline water (Table 1). Germination was performed using fresh water and algae water extract (5-cm plastic pots). All of the transplants were irrigated by saline water (2, 4, and 7 dSm/1) during greenhouse growth.
All pots containing the tomato transplants were transferred outside the greenhouse till the best growth was chosen (7 dSm/1), then transferred to the clayey soil, irrigated by saline water, and different algae (Chlorella, Spirulina, and their mixture) water extract. The pots were irrigated using depletion method that calculate irrigation amount by weighing the container’s the following equation was used [37, 38].
The pots containing seedlings sprouted from seeds soaked in microalgae extracts and were also fertilized by 1% extracts of Chlorella vulgaris, Spirulina platensis, and Chlorella:Spirulina mixture in the form of foliar spraying (15 ml) and applied to soil (50 ml). This dose is commercially advised especially with young plants (transplants) and is recommended to be more economical [6].
Each treatment had three replicates; on the other hand, control pots were irrigated with saline water only with no algae application. After 10 weeks, certain growth parameters, including plant height, leaf area, and wet and dry weights were measured in addition to the determination of chlorophyll a and b concentrations, according to [39].
Second, transplanting in clayey soil was performed in an experimental research area of the National Water Research Center until the final yield was obtained. The grown transplants were periodically irrigated (48 h) with 7.0 dS m−1 saline water and fertilized by using the microalgae water extracts (1%) as foliar spraying, while for soil, a commercial organic microalgae biofertilizer (purchased from National Research Center, Algal Biotechnology Unit) was added to irrigation water (7 ml/10 l), until the final yield is obtained.
2.3 Yield and fruit quality
Weekly two to three times, fruits were harvested at the end of the harvesting season, and the total fruit yield and average fruit weight were determined. Concerning fruit weight loss, two tomato fruits from each treatment were weighed using a 0.0 to 5.0 kg ± 0.01 g precision scale, and the value was reported on an initial weight basis. Tomato fruits were analyzed for micronutrients (Fe, Zn, Mn, and Cu) after dry ash and acid solubilization. Ash was achieved at 500 °C in a muffle furnace, and the obtained ash was dissolved in 2.0 NHCl (50 ml) and then filtered over ashless filter paper [34]. Micronutrients were measured using inductively coupled plasma-optical emission spectroscopy (ICP-OES) with an ultrasonic nebulizer (USN). The ICP instrument was a Perkin Elmer Optima 3000, USA.
2.4 Statistical analysis
The experimental design used was a split-plot design with three treatments and three replicates, and the data were statistically analyzed using analysis of variance (SPSS, version 10.0). All obtained data were subjected to statistical analysis of variance, according to [40] and means separation was carried out according to the least significant difference (LSD) and [41] at 5% levels of probability.
3 Results
The approximate biochemical composition, ash content, and water extract mineral content of outdoor-produced Chlorella vulgaris and Spirulina platensis are shown in Tables 2 and 3. Spirulina surpasses Chlorella in concern crude protein; consequently, the initial amino acids, carbohydrates, carotene, and ash content; however, ether extract (oils) was higher in Chlorella vulgaris. In spite of the initial content of extract, the individual fraction might enhance the treated plants by their extract rather than content. Otherwise, the exorability potential of Spirulina platensis is more than that of Chlorella vulgaris due to the difference in cell wall structure of the used algae, where Spirulina cell wall is more bio-degradable than Chlorella.
3.1 Enhancement effect of microalgae as biofertilizers on growth parameters of tomato plants under salinity stress
Many growth factors could be obtained in marked quantities from the used algae; however, they exhibited slight differences due to their natural taxa. They include amino acids, short-chain peptides, polysaccharides, and organic acids. Such compounds were considered strong chelating agents. Furthermore, sufficient mineral content was also found in a concentration that reacted as a nanoparticle and was mostly coupled with organic compounds. Water extracts of microalgae Spirulina platensis, Chlorella vulgaris, and their mixture were used as biostimulators for growing tomato seeds irrigated with saline water at three salinity levels, 2, 4, and 7 dS/m under greenhouse conditions until reaching the seedling stage. The data presented in Table 4 show that the application of microalgae to soil and as foliar spraying combined with saline water irrigation results in a noticeable increase in tomato transplant growth parameters, including plant height, leaf area, and wet and dry weights. These increases were observed in all tomato plants treated with different microalgae water extracts and different salinity levels, compared with the control plants, where no algae were applied. There was a retarding effect on control plant growth with increasing salinity levels, and the highest growth rate was detected at 2 dS/m (plant height: 26.5 cm; leaf area: 62.7 cm2; fresh weight: 4.55 g; and dry weight: 0.38 g). The lowest growth rate was observed at 7 ds/m (plant height: 24.0 cm; leaf area: 23.5 cm2; fresh weight: 3.0 g; and dry weight: 0.21 g). In contrast, tomato plants treated with algal water extracts showed elevated growth rates under saline water irrigation, where the studied growth parameters continued to develop from 2 to 7 ds/m salinity levels, indicating that microalgae fertilization improved tomato tolerance and enhanced its ability to grow and progress under salinity stress.
Furthermore, the data in Table 4 show that at the high salinity margin used (7 dS/m), the highest growth rates are determined in tomato plants treated with the Spirulina:Chlorella water extract mixture (plant height: 28.5 cm; leaf area: 75.6 cm2; fresh weight: 5.53 g; and dry weight: 0.42 g), followed by Spirulina platensis water extract (plant height: 26.0 cm; leaf area: 64.6 cm2; fresh weight: 4.26 g; and dry weight: 0.34 g), and then by Chlorella vulgaris water extract (plant height: 26.0 cm; leaf area: 61.7 cm2; wet weight: 4.09 g; and dry weight: 0.32 g). Due to the promising results observed in growth parameters at saline water irrigation of 7 ds/m combined with algae fertilization, the rest of the experiments in the present study proceeded at the 7 ds/m salinity level.
Statistical analysis (Table 4) also revealed that all vegetative characteristics increased significantly in response to microalgae fertilization (see the attached Tables a–d).
3.2 Effect of microalgae on chlorophyll content
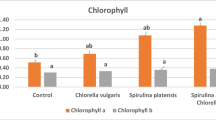
The data illustrated in Fig. 1 show that the chlorophyll (a and b) contents are improved in stressed tomato plants treated with the Spirulina:Chlorella mixture water extract by more than 70.0%, followed by Spirulina platensis water extract (> 50.0%) and Chlorella vulgaris water extract (> 18.0%), in comparison with the control. The chlorophyll a contents were significantly higher at Spirulina:Chlorella mixture water extract too, followed by Spirulina platensis water extract and Chlorella vulgaris water extract; whereas, there was no significant difference for chlorophyll b which were of the same contents at all treatments.
3.3 Effect of microalgae on tomato yield
Tomato plants were transplanted in clayey soil at the National Water Research Center (NWRC) research station to study the effect of microalgae extract treatments with organic microalgae biofertilizer on tomato yield under saline irrigation (7 dS/m). Figure 2 illustrates the application of Spirulina platensis water extract significantly increases tomato yield (2.93 kg/plant), followed by Spirulina:Chlorella mixture (1.82 kg/plant), then by Chlorella vulgaris (1.22 kg/plant), while the lowest yield is noticed in control plants (0.70 kg/plant).
3.4 Nutritional status of tomato fruits as affected by saline irrigation and algae fertilization
In the present study, some micronutrients (Fe, Zn, Mn, and Cu) were determined in produced tomato fruits to evaluate whether the accumulation of such nutrients could increase the nutritive value of the fruits in addition to their physiological functions in fruits (Table 5). All of the determined micronutrients were markedly accumulated by the produced tomato fruits due to algal treatments; however, the tomato plants were grown in relatively saline water (7.0 dS·m−1). Due to seed soaking and among the algal extracts applied, Chlorella extract surpassed all other treatments, especially in iron (Fe) accumulation.
Data in Table 5 illustrate that the application of algae extracts might lead to reducing the adverse saline irrigation effect on tomato plants by improving the roots’ ability to absorb more nutrients, not only macro- but also micronutrients, compared with control plants.
3.5 Effect of salinity and algae fertilization on fruit weight loss
The results in Fig. 3 show that the foliar spraying effect of microalgae extracts and soil fertilization with organic microalgae biofertilizer under salinity stress (7 dS/m) on experimental tomato has a considerable effect on tomato fruit weight loss % at room temperature with all treatments. The lowest weight loss % was with Chlorella treatment, compared with other treatments. However, weight loss % increases in the absence of algae, compared with the control. The differences between the four treatments were significant, except the differences between Spirulina, and the mixture was not significant. The tomato fruits of Chlorella and mixture treatments only stay for 45 days%, where the control and the treatment with Spirulina only stay for 30 days.
4 Discussion
From the above results, algal is improving plant growth of studied tomato, so Chlorella cell wall must break to free its metabolites which make them available to utilize [30]. The microfibrillar layer (chitosan-like layer) of Chlorella cell wall is composed of glucosamine [42]. In the mature stage, due to the presence of a sporopollenin layer, cell wall thickness explaine its rigidity [43, 44]. The cell organization of the filamentous microalga (Arthrospira platensis) is typical of a prokaryote Gram‐negative bacterium, lacking membrane‐bound organelles. The cell wall constitutes an envelope composed by several layers, mostly of peptidoglycan and lipopolysaccharide nature [45]. For this reason, there is no doubt that algal cell wall composition affects the exorability potential and the subsequent effect on plant growth which is improving. One of the main considerations is the presence of a huge amount of natural phytohormones that surpass all natural sources [46, 47], as shown in Tables 1 and 2; both algae were found to be rich in nutrients and biologically active constituents, which are expected to enhance general plant growth and overcome salinity stress.
Moreover, biostimulants act on plant physiology through different pathways, improving crop growth, yield, quality, uptake of nutrients, tolerance to abiotic stresses, and shelf life of the harvest [13, 15]. The implemented growth conditions of both algae led to the biodegradation of cell metabolites to a simple form, mainly short-chain amino acids and organic acids, which in turn increased the biochelation of minerals and nutrients. These conditions also obligate algae to accumulate large amounts of phytohormones and antioxidants. Biochelation allows nutrients to be more efficient in plant nutrition and increase plant resistance to abiotic stress, including salinity. It could be concluded that the individual algae extract effect on tomato plant growth goes back to several specific chemical and biochemical components. First, the algae were grown early under drastic macro- and micronutrient addition, which allowed the accumulation of nanonutrients.
Spirulina platensis extract resulted in higher vegetative growth, chlorophyll content, and chemical characteristics under both the absence and presence of salinity stress; in addition, it was found to enhance the total yield of salt-sensitive cv. Carmen tomato plants by approximately 30% under hypersaline conditions [2]. Chlorophyll is the principal material for photosynthesis and is found in plants, algae, and bacteria, giving these organisms their green color in addition to allowing them to absorb light energy for photosynthesis. Chlorophyll content reveals the photosynthetic ability of leaves and plant health [48]. Another growth experiment with tomatoes carried out by [27] proved that microalgae fertilization increased tomato plants’ chlorophyll content by 12%, compared to control plants without fertilizers. In this context, the appreciable influence of alga extract may be due to its effect on increasing cell membrane permeability and plant efficiency in promoting the absorption of nutrients, as well as its role in delaying the aging of leaves by reducing chlorophyll degradation [6]. Chlorophyll a and b were considered as a physiological parameter at 7.0 dS·m−1 of salinity level. The effect was obviously noticed on chlorophyll a as the principle photosynthetic pigment. However, salinity also adversely affected the shoot dry weight, leaf area, leaf chlorophyll content, and also fruit weight/plant mostly at 8 dS/m [49]; the increasing chlorophyll content could be ascribed to the effect of algal biostimulators. There is a close relationship between leaf area and photosynthetic pigment concentration, where the concentrations of chlorophyll (a and b) are increasing, along with carotenoids. This is found to be in agreement with [50, 51] who mentioned that the increasing photosynthetic pigment breakdown or decreased production were reported as salinity harmful effect.
Meanwhile, using algae extracts led to overcoming the adverse effects of salinity by significantly increasing the yield of plants, especially with Spirulina, which could be attributed to the occurrence of micro- and macronutrients, growth hormones, vitamins, amino acids, and other ingredients in algae that might be responsible for better enhancement in yield production [2, 28]. Algae increase the uptake and accumulation of elements in plants due to its rich source of potassium and contains considerable amounts of Ca, Cu, Fe, Mg, Mn, P, and Zn, which explains the significant increase in vegetative growth and yield, and hence the contents of nitrogen, phosphorus, and protein in leaves and leaf chlorophyll content for most crops [52]. Algae extracts might lead to reducing the adverse saline irrigation effect on tomato plants by improving the roots’ ability to absorb more nutrients, not only macro- but also micronutrients, compared with control plants. This could be attributed to the constituents of phytohormones and natural growth regulators found in algae extracts, which played a major role in the physiological plant performance [2, 30]. Additionally, [53] found that using a mixture of natural elements and alga Amphora coffeaeformis extract mitigated the disadvantageous effect of salinity stress on pear trees and improved fruit chemical properties as well as macro- and micronutrient contents. The effect of Chlorella vulgaris on micronutrient accumulation might be related to its natural habitat as a freshwater green alga rich in growth regulators that is more responsive to salinity stress than Spirulina platensis, which grows in brackish water.
However, weight loss % increases in the absence of algae, compared with the control. The same results were obtained by [54, 55].
Algae well considered as plant macronutrients, micronutrients, and number of key bioactive chemicals supplier including phytohormones, amino acids, and vitamins making a strong candidate against biotic and abiotic stress factors. Such higher micronutrient content improves oxidative stress tolerance through promoting of antioxidant enzyme synthesis, which helps to alleviate salt stress through their radical scavenging activity and also through enhancing micronutrient movement and distribution within the entire plant [56,57,58]. Algal biostimulators or algal extract use led to re-stabilizing the membranes, reducing ion leakage, increasing the water retention capabilities of the salt-stressed plants by increasing the water-withholding capability [59].
5 Conclusion
The results show that studied tomato plants treated by the Spirulina:Chlorella mixture, followed by Spirulina platensis and Chlorella vulgaris water extracts under the experimental salinity levels (2, 4, and 7 dS·m/1) gave the highest transplant vegetative growth and chlorophyll content. In addition, the highest tomato yield of the successive grown tomato transplant at 7.0 dS/cm was determined in tomato plants treated with the mixture extract. Both algal extracts and their mixture enhanced the bioaccumulation of micronutrients (Fe, Zn, Mn, and Cu), compared with the control, while Chlorella extract surpassed Spirulina and mixture extracts. The normal percentage of algal bioash reached a maximum of 12% on a dry weight basis. The best algal extract to use at saline conditions was Spirulina:Chlorella mixture, followed by Spirulina platensis and Chlorella vulgaris, whereas for shelf-life Chlorella vulgaris gave the best results. Finally, the study has positive results, specially for water scarcity problem where using (7 dS·m/1) to irrigate tomato is considered as a good performance for using this range of saline water to irrigate tomato as a one of the strategic crops. The other positive results that can help in solving the tomatoes’ shelf-life problems, the results noticed the experimental tomato stayed at room temperature for 45 days with the Chlorella vulgaris treatment. In addition, till now there are no negative results, but the authors are still working and also may be in the future.
Data availability
The data that support the findings of this study are available upon request.
References
Hammam AA, Mohamed ES (2020) Mapping soil salinity in the East Nile Delta using several methodological approaches of salinity assessment. Egypt J Remote Sens Space Sci 23:125–131. https://doi.org/10.1016/j.ejrs.2018.11.002
El-Sayed AB, Shehata AS, Taha SS, Hamouda HA, Abdelgawad KF, Youssef DM (2018) Algae extract overcoming the adverse effects of saline stress in hydroponic grown tomato plants. J Food Agric Environ 16(2):92–99 (https://www.cabdirect.org/cabdirect/abstract/20193060296)
Mohamed NN (2017) The Nile Delta. The handbook of Environmental Chemistry, vol 55. https://doi.org/10.1007/978-3-319-56124-0
Wang N, Zhao Z, Zhang X, Liu S, Zhang K, Hu M (2023) Plant growth, salt removal capacity, and forage nutritive value of the annual euhalophyte Suaeda salsa irrigated with saline water. Front Plant Sci 13:1040520. https://doi.org/10.3389/fpls2022.1040520
Surendran U, Sandeep O, Joseph EJ (2016) The impacts of magnetic treatment of irrigation water on plant, water and soil characteristics. Agric Water Manag 178:21–29. https://doi.org/10.1016/j.agwat.2016.08.016
Enan SAAM, El-Saady AM, El-Sayed AB (2016) Impact of foliar feeding with alga extract and boron on yield and quality of sugar beet grown in sandy soil. Egypt J of Agron 38(2):319–336. https://doi.org/10.21608/AGRO.2016.622
Khalil AA, Fetyan NA, Hemeid NM (2010) Effect of bacillus circulans and azotobacter chroococcum inoculation on potato production in presence of different mineral potassium sources. J Agric Chem Biotechnol 1(9):471–483. https://doi.org/10.21608/JACB.2010.90059
Hassan HBA, Ahmed EAE (2018) Economic efficiency of tomato production in Fayoum governorate, Egypt. Middle East J Agric Res 7(4):1798–1810 (https://www.curresweb.com/mejar/mejar/2018/1798-1810.pdf)
Rachidi F, Benhima R, Sbabou L, El Arroussi H (2020) Microalgae polysaccharides biostimulating effect on tomato plants: growth and metabolic distribution. Biotechnol Rep 25:e00426. https://doi.org/10.1016/j.btre.2020.e00426
Suganya T, Varman M, Masjuki HH, Renganathan S (2016) Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renew Sustain Energy Rev 55:909–941. https://doi.org/10.1016/j.rser.2015.11.026
Garcia-Gonzalez J, Sommerfeld M (2016) Biofertilizer and bio stimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061. https://doi.org/10.1007/s10811-015-0625-2
Santos VB, Araújo AS, Leite LFC, Nunes LL, Melo WJ (2012) Soil mirobial biomass and organic matter fractions during transition from conventional to organic farming systems. Geoderma 170:227–231. https://doi.org/10.1016/j.geoderma.2011.11.007
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14. https://doi.org/10.1016/j.scienta.2015.09.021
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:2049. https://doi.org/10.3389/fpls.2016.02049.eCollection2016
Rouphael Y, Colla G (2018) Synergistic biostimulatory action: designing the next generation of plant biostimulants for sustainable agriculture. Front Plant Sci 9:1655. https://doi.org/10.3389/fpls.2018.01655
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Görs M, Schumann R, Hepperle D, Karsten U (2010) Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J Appl Phycol 22:265–276. https://doi.org/10.1007/s10811-009-9455-4
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria: a precious bioresource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529. https://doi.org/10.3389/fmicb.2016.00529
Osman MEH, Abo-Shady AM, El-Nagar MMF (2016) Cyanobacterial Arthrospira (Spirulina platensis) as safener against harmful effects of fusilade herbicide on faba bean plant. Rend Fis Acc Lincei 27:455–462. https://doi.org/10.1007/s12210-015-0498-y
Nawrocka D, Kornicka K, Śmieszek A, Marycz K (2017) Spirulina platensis improves mitochondrial function impaired by elevated oxidative stress in adipose-derived mesenchymal stromal cells (ASCs) and intestinal epithelial cells (IECs), and enhances insulin sensitivity in equine metabolic syndrome (EMS) horses. Mar Drugs 15:237. https://doi.org/10.3390/md15080237
Bileva T (2013) Influence of green algae Chlorella vulgaris on infested with xiphinema index grape seedlings. J Earth Sci Clim Chang 4(2):136. https://doi.org/10.4172/2157-7617.1000136
Grzesik M, Romanowska-Duda Z (2015) Ability of cyanobacteria and green algae to improve metabolic activity and development of willow plants. Pol J Environ Stud 24(3):1003–12. https://doi.org/10.15244/pjoes/34667
Hosseini MK, Powell AA, Bingham IJ (2003) The interaction between salinity stress and seed vigor during germination of soybean seeds. Seed Sci Technol 31:715–725. https://doi.org/10.15258/sst.2003.31.3.20
Epstein E, Norlyn JD, Rush DW (1980) Saline culture of crops: a genetic approach. Science 210(4468):399–404
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71. https://doi.org/10.1126/science.210.4468.399
Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A (2019) Microalgal biostimulants and biofertilizers in crop productions. Agronomy 9(192178):21–29. https://doi.org/10.3390/agronomy9040192
Jimenez R, Markou G, Tayibi S, Barakat A, Chapsal C, Monlau F (2020) Production of microalgal slow-release fertilizer by valorizing liquid agricultural digestate: growth experiments with tomatoes. Appl Sci 10:3890. https://doi.org/10.3390/app10113890
Coppens J, Grunert O, Van Den Hende S, Vanhoutte I, Boon N, Haesaert G, De Gelder L (2016) The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J Appl Phycol 28:2367–2377. https://doi.org/10.1007/s10811-015-0775-2
Gitau MM, Farkas A, Ord V, Maroti G (2022) Evaluation of the biostimulant effects of two Chlorophyta microalgae on tomato (Solanum lycopersicum). J Clean Prod 364:132689. https://doi.org/10.1016/j.jclepro.2022.132689
Abdel-Maguid AA, El-Sayed AB, Hassan HSA (2004) Growth enhancement of olive transplants by broken cells of fresh green algae as soil application Minufiya. J Agric Res 29(3):723–733. https://doi.org/10.21608/ejss.2021.99643.1472
El-Sayed AEB, Abdalla FE, Abdel-Maguid AWA (2001) Use of some commercial fertilizer compounds for Scenedesmus cultivation. Egypt J Phycol 2:9–16. https://doi.org/10.21608/egyjs.2001.113268
Zarrouk C (1966) Contribution a l’etude d’une cyanophyceee: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima (Setch et Gardner) Geitler. PhD Thesis, Universite de Paris
Stanier RY, Kunisawa R, Mandel M et al (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35(2):171–205
Methods of Analysis for Soils, Plants and Waters. Univ. California Div. Agric. Sci. Priced Publication, Oakland. https://doi.org/10.2136/sssaj1963.03615995002700010004x
Rosni S, Ahmad F, Awang A (2015) Crude proteins, total soluble proteins, total phenolic contents and SDS-PAGE profile of fifteen varieties of seaweed from Semporna, Sabah, Malaysia. Int Food Res J 22:1483–1493
Nagata M, Yamashita I (1992) Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaish 39(10):925–928. https://doi.org/10.3136/nskkk1962.39.925
Israelsen OW, Hanson VE (1962) Irrigation, principles and practices, 3rd edn. Wiley International Edition New York, 279
Shawky I, Rawash MA, Behairy Z, Bondok M, Mostafa M (1997) Growth and chemical composition of grape transplants as affected by some irrigation regimes. Acta Hortic 441:439–42448. https://doi.org/10.17660/ActaHortic.1997.441.69
Von-Wensttein DV (1957) Chlorophyll letal and Der supunikors kapisene. Jor Winneck Sec Der Plastiden Exp Cell Res 12:427. https://doi.org/10.1016/0014-4827(57)90165-9
Snedecor GW, Cochran WG (1980) Statistical methods 7th edition Iowa State University Press, Ames, Iowa, USA p 507
Duncan D B (1958) Multiple range and multiple F test. Biometrics 11:1–42. https://doi.org/10.2307/3001478
Yvonne N, Tomas K (2000) Cell wall development, microfibril and pyrenoid structure in type strains of Chlorella vulgaris, C. kessleri, C. sorokiniana compared with C. luteoviridis (Trebouxiophyceae, Chlorophyta). Arch Hydrobiol 100:95–105. https://doi.org/10.1127/algol_stud/100/2000/95
Atkinson AW Jr, Gunning BES, John PCL (1972) Sporopollenin in the cell wall of Chlorella and other algae: ultrastructure, chemistry, and incorporation of 14C-acetate, studied in synchronous cultures. Planta 107:1–32. https://doi.org/10.1007/BF00398011
Burczykj HM (1981) The ultrastructure of the outer cell wall-layer of Chlorella mutants with and without sporopollenin. Plant Syst Evol 138:121–137. https://doi.org/10.1007/BF00984613
Costerton JW, Ingram JM, Cheng KJ (1974) Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev 38:87. https://doi.org/10.1128/br.38.1.87-110.1974
Lafargaa T, Acién-Fernándeza FA, Garcia-Vaquero M (2020) Bioactive peptides and carbohydrates from seaweed for food applications: natural occurrence, isolation, purification, and identification. Algal Res 45:101909. https://doi.org/10.1016/j.algal.2020.101909
Zamljen T, Hudina M, Veberič R, Slatnar A (2021) Biostimulative effect of amino acids and green algae extract on capsaicinoid and other metabolite contents in fruits of Capsicum spp. Chem Biolog Technol Agric 8:63. https://doi.org/10.1186/s40538-021-00260-5
Zulkarnaini ZM, Sakimin SZ, Mohamed MTM, Jaafar HZE (2019) Relationship between chlorophyll content and soil plant analytical development values in two cultivars of fig (Ficus carica L.) as brassinolide effect at an open field. Earth Environ Sci 250:012025. https://doi.org/10.1088/1755-1315/250/1/012025
Parvin H, Ahmed UK, Islam MM, Haque MdN (2015) Response of tomato plant under salt stress: role of exogenous calcium. J Plant Sci 10(6):222–233. https://doi.org/10.3923/jps.2015.222.233
Mutale-joan C, Rachidi F, Mohamed HA, Mernissi NE, Aasfar A, Barakate M, Arroussi HE (2021) Microalgae-cyanobacteria–ed biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J Appl Phycol 33:3779–3795. https://doi.org/10.1007/s10811-021-02559-0
Cao K, Yu J, XuD Ai K, Bao E, Zou Z (2018) Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol 18:92. https://doi.org/10.1186/s12870-018-1310-9
Abdel-Maguid AA, El-Fouly MM, El-Sayed AB (2005) Use of algae on plant nutrition a review article the 3rd Conference of Agricultural and Biological Research Division, Prospects of the Recent Agriculture Research April 21–23 2005 NRC Cairo Egypt
Mansour N, Ataya S (2021) Improving productivity of “Le-Conte” pear trees grown in new reclaimed soils using natural elements mixture and algae extract. Egypt J Hortic 48(2):221–239. https://doi.org/10.21608/ejoh.2021.75930.1174
Magán JJ, Gallardo M, Thompson RB (2008) Effects of salinity on fruit yield and quality of tomato grown in soil-less culture in green houses in Mediterranean climatic conditions. Agric Water Manag 95(9):1041–1055. https://doi.org/10.1016/j.agwat.2008.03.011
Fallik E, Alkalai-Tuvia S, Chalupowicz D, Zaaroor-Presman M, Offenbach R, Cohen S, Tripler E (2019) How water quality and quantity affect pepper yield and postharvest quality. Horticulture 5(1):4. https://doi.org/10.3390/horticulturae5010004
Stanley-Raja V, Senthil-Nathan S, Chanthini KMP, Sivanesh H, Ramasubramanian R, Karthi S, Shyam-Sundar N, Vasantha-Srinivasan P (2021) Kalaivani K (2021) Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci Rep 11:20488. https://doi.org/10.1038/s41598-021-99391-w
El-Katony TM, Deyab MA, El-Adl MF, Ward FME-N (2021) Extracts of the brown alga Dictyota dichotoma (Hudson) JV Lamouroux alleviate salt stress in rice (Oryza sativa L.) during germination. J Plant Growth Regul 40:986–999. https://doi.org/10.1007/s00344-020-10156-7
Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric 4:1–12. https://doi.org/10.1186/s40538-017-0089-5
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48. https://doi.org/10.1016/j.scienta.2015.09.012
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The others and Maryam M. Mostafa: application of statistical data; review of post-publication stages. Maryam M. Mostafa, Doaa M. Hammad, and Marwa M. Reda are putting the ideas of overarching research goals and the design methodology; helped in conducting the research investigation process, wrote the main manuscript text, and prepared the figures and tables. Abo El-Khair B. El-Sayed: preparation of microalgae and their extracts as well as their analysis, revision of the data, preparation and presentation of the published research, and review of the research prepublication. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Applicable for both human and/ or animal studies: NA. Ethical committees, Internal Review Boards and guidelines followed must be named: NA.
Consent to participate and consent to publish
NA
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafa, M.M., Hammad, D.M., Reda, M.M. et al. Water extracts of Spirulina platensis and Chlorella vulgaris enhance tomato (Solanum lycopersicum L.) tolerance against saline water irrigation. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04460-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04460-x