Abstract
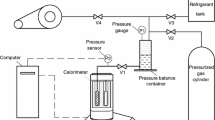
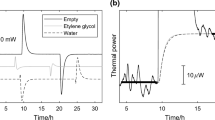
This article includes the results of experimental research, determining specific heat of water, glycerin, rapeseed oil and aqueous solutions of carboxymethylcellulose sodium salt. Two research methods were used during measurements. The first one uses Newton’s law of cooling. The second one uses electrocalorimetric measurements. The average calculated values of determined parameter for model liquids did not exceed 1 % error in cooling method and did not exceed 2 % error in calorimetric method. Good conformity of both methods enabled to use them in measurements of liquids with unknown values of specific heat C. In case of carboxymethylcellulose sodium salt, together with increasing concentration, specific heat increases. Significant impact on obtained results has also molar mass M. The highest values C, were obtained for carboxymethylcellulose sodium salt with a molar mass 700,000 kg/kmol and concentration 0.005 kg p/kg within the range of temperatures (303–325) K. Obtained result was over 19 % higher than water.
Similar content being viewed by others
References
Cho Y.I., Hartnett J.P.: Non-Newtonian fluids in circular pipe flow. Adv. Heat Transf. 15, 59–141 (1982)
Metzner A.B.: Heat transfer in non-Newtonian fluids. Adv. Heat Transf. 2, 357–397 (1965)
Bellet D., Singelin M., Thirriot C.: Determination des proprietes thermophysiques des liquides non-Newtoniens a l‘aide d‘une cellule a cylinders coaxiaux. Int. J. Heat Mass Transf. 18, 1177–1186 (1975)
Raynaud, M.; Bransier, J.; Delaunay, D.: Fluides complexes. Détermination de leur conductivité thermique et de leur capacité thermique volumique. Rev. Gén. Therm. Fr., 279, 241 (1985)
Semmar N., Tanguier J.L., Rigo M.O.: Specific heat of carboxymethyl cellulose and carbopol aqueous solutions. Termochim. Acta 402, 225–235 (2003)
Semmar N., Tanguier J.L., Rigo M.O.: Analytical expressions of specific heat capacities for aqueous solutions of CMC and CPE. Termochim. Acta 419, 51–58 (2004)
Ramaswamy H.S., Zareifard M.R.: Evaluation of factors influencing tube-flow fluid-to-particle heat transfer coefficient using a calorimetric technique. J. Food Eng. 45, 127–138 (2000)
Villano P., Carewska M., Passerini S.: Specific heat capacity of lithium polymer battery components. Termochim. Acta 402, 219–224 (2003)
Stoliarov S.I., Walters R.N.: Determination of the heats of gasification of polymers using differential scanning calorimetry. Polym. Degrad. Stab. 93, 422–427 (2008)
Kato H., Sasaki K.: Avoiding error of determining the martensite finish temperature due to thermal inertia in differential scanning calorimetry: model and experiment of Ni–Ti and Cu–Al–Ni shape memory alloys. J. Mater. Sci. 47, 1399–1410 (2012)
Kwarciak J., Morawiec H.: Some interpretation problems of thermal studies of the reversible martensitic transformation. J. Mater. Sci. 23, 551–557 (1988)
Milkereita B., Becka M., Reicha M., Kesslera O., Schickb C.: Precipitation kinetics of an aluminium alloy during Newtonian cooling simulated in a differential scanning calorimeter. Termochim. Acta 522, 86–95 (2011)
Kempen A.T.W., Sommer F., Mittemeijer E.J.: Calibration and desmearing of differential thermal analysis measurement signal upon heating and cooling. Termochim. Acta 383, 21–30 (2002)
Perry J.H., Chilton C.H.: Chemical Engineers’ Handbook. McGraw Hill, New York (1973)
Broniarz-Press L, Agaciński P, Różański J: Fluidization in polymer solutions and its effect on rheological properties of a fluid. Appl. Mech. Eng. 4, 197–202 (1999)
Rozanski J., Broniarz-Press L., Dryjer S., Bednarz J.: Effect of the strong electrolyte addition on rheological properties of aqueous solutions of sodium carboxymethyl cellulose. Int. J. Appl. Mech. Eng. 8, 207–212 (2003)
Harison G.M.: A note on the effect of polymer rigidity and concentration on spray atomization. J. Non-Newton. Fluid Mech. 85, 93–104 (1999)
Broniarz-Press L., Prałat K.: Thermal conductivity of Newtonian and non-Newtonian liquids. Int. J. Heat Mass Transfer 52, 4701–4710 (2009)
Prałat, K.: Determining specific heat of aqueous solutions of the sodium salt carboxymethylcellulose with electrocalorimetric method. In z. Ap. Chem., 54, nr 1, 14–15 (2015) – (in Polish)
Heim, D.; Mrowiec, A.; Prałat, K.: Analysis and interpretation of results of thermal conductivity obtained by the hot wire method. Exp. Techniques (2014). doi:10.1111/ext.12092
Osborne J.; Stimson M.; Ginnings S.: B of S. Jour. Res. 23, 238 (1939). In: Handbook of Chemistry and Physics, 53rd edn. Cleveland, Ohio, D128 (1972–1973)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prałat, K. Comparison of Electrocalorimetric and Cooling Methods to Determine Specific Heat of Aqueous Solutions of the Sodium Salt Carboxymethylcellulose. Arab J Sci Eng 40, 3409–3415 (2015). https://doi.org/10.1007/s13369-015-1858-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-015-1858-8