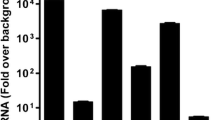
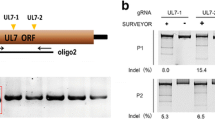
Abstract
The herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) encodes several microRNAs. One of these, miR-H2, overlaps and is antisense to the ICP0 gene and appears to decrease expression of the ICP0 protein. To determine if miR-H2 plays a role in the HSV-1 latency-reactivation cycle, we constructed a mutant, McK-ΔH2, in which this microRNA has been disrupted without altering the predicted amino acid sequence of ICP0. McK-ΔH2 produced increased amounts of ICP0. Although replication of McK-ΔH2 was similar to that of its wild-type (wt) McKrae parental virus in RS cells and mouse eyes, McK-ΔH2 was more neurovirulent in Swiss-Webster mice than McKrae based on the percent of mice that died from herpes encephalitis following ocular infection. In addition, using a mouse trigeminal ganglia (TG) explant model of induced reactivation, we show here for the first time that miR-H2 appears to play a role in modulating HSV-1 reactivation. Although the percent of TG from which virus reactivated by day 10 after explant was similar for McK-ΔH2, wt McKrae, and the marker-rescued virus McK-ΔH2Res, at earlier times, significantly more reactivation was seen with McK-ΔH2. Our results suggest that in the context of the virus, miR-H2 downregulates ICP0 and this moderates both HSV-1 neurovirulence and reactivation.










Similar content being viewed by others
References
Ahmed M, Lock M, Miller CG, Fraser NW (2002) Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J Virol 76:717–729
Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H (2011) The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol 85:4184–4197, PMC:3126262
Allen SJ, Rhode-Kurnow A, Mott KR, Jiang X, Carpenter D, Rodriguez-Barbosa JI, Jones C, Wechsler SL, Ware CF, Ghiasi H (2014) Interactions between herpesvirus entry mediator (TNFRSF14) and latency-associated transcript during herpes simplex virus 1 latency. J Virol 88:1961–1971, PMC:3911542
Block TM, Deshmane S, Masonis J, Maggioncalda J, Valyi-Nagi T, Fraser NW (1993) An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology 192:618–630
Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L (2011) The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol 85:9127–9138, PMC:3165846
Chentoufi, A.A., Dervillez, X., Dasgupta, G., Nguyen, C., Kabbara, K.W., Jiang, X., Nesburn, A.B..., Wechsler, S.L., and Benmohamed, L. 2012. The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol
Du T, Zhou G, Roizman B (2011) HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc Natl Acad Sci U S A 108:18820–18824, PMC:3219146
Du T, Zhou G, Roizman B (2012) Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci U S A 109:14616–14621, PMC:3437834
Flores O, Nakayama S, Whisnant AW, Javanbakht H, Cullen BR, Bloom DC (2013) Mutational inactivation of herpes simplex virus 1 microRNAs identifies viral mRNA targets and reveals phenotypic effects in culture. J Virol 87:6589–6603, PMC:3676078
Herpetic Eye Disease Study and Group (1998) Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group [see comments]. N Engl J Med 339:300–306
Hill JM, Sedarati F, Javier RT, Wagner EK, Stevens JG (1990) Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117–125
Hjalmarsson A, Blomqvist P, Skoldenberg B (2007) Herpes simplex encephalitis in Sweden, 1990-2001: incidence, morbidity, and mortality. Clin Infect Dis 45:875–880
Inman M, Perng GC, Henderson G, Ghiasi H, Nesburn AB, Wechsler SL, Jones C (2001) Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J Virol 75:PMC:114855–3646
Jaber T, Workman A, Jones C (2010) Small noncoding RNAs encoded within the bovine herpesvirus 1 latency-related gene can reduce steady-state levels of infected cell protein 0 (bICP0). J Virol 84:6297–6307, PMC:2903259
Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, BenMohamed L, Fraser NW, Jones C, Wechsler SL (2011) The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J Virol 85:2325–2332, PMC:3067767
Jin L, Perng GC, Mott KR, Osorio N, Naito J, Brick DJ, Carpenter D, Jones C, Wechsler SL (2005) A herpes simplex virus type 1 mutant expressing a baculovirus inhibitor of apoptosis gene in place of latency-associated transcript has a wild-type reactivation phenotype in the mouse. J Virol 79:12286–12295
Jin L, Perng GC, Carpenter D, Mott KR, Osorio N, Naito J, Brick DJ, Jones C, Wechsler SL (2007) Reactivation phenotype in rabbits of a herpes simplex virus type 1 mutant containing an unrelated antiapoptosis gene in place of latency-associated transcript. J Neurovirol 13:78–84
Jin L, Carpenter D, Moerdyk-Schauwecker M, Vanarsdall AL, Osorio N, Hsiang C, Jones C, Wechsler SL (2008) Cellular FLIP can substitute for the herpes simplex virus type 1 latency-associated transcript gene to support a wild-type virus reactivation phenotype in mice. J Neurovirol 14:389–400, PMC:2980827
Leib DA, Bogard CL, Kosz-Vnenchak M, Hicks KA, Coen DM, Knipe DM, Schaffer PA (1989) A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol 63:2893–2900
Nesburn AB (1983) Report of the corneal disease panel: vision research: a national plan 1983-1987, vol II, Part III., II, part III. C.V. Mosby Co., St. Louis
Pan D, Flores O, Umbach JL, Pesola JM, Bentley P, Rosato PC, Leib DA, Cullen BR, Coen DM (2014) A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency. Cell Host Microbe 15:446–456, PMC:4142646
Peng W, Jin L, Henderson G, Perng GC, Brick DJ, Nesburn AB, Wechsler SL, Jones C (2004) Mapping herpes simplex virus type 1 latency-associated transcript sequences that protect from apoptosis mediated by a plasmid expressing caspase-8. J Neurovirol 10:260–265
Peng W, Henderson G, Inman M, BenMohamed L, Perng GC, Wechsler SL, Jones C (2005) The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal Ganglia of acutely infected mice. J Virol 79:6162–6171
Perng GC, Dunkel EC, Geary PA, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL (1994) The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol 68:8045–8055
Perng GC, Thompson RL, Sawtell NM, Taylor WE, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL (1995) An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J Virol 69:3033–3041
Perng GC, Chokephaibulkit K, Thompson RL, Sawtell NM, Slanina SM, Ghiasi H, Nesburn AB, Wechsler SL (1996a) The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J Virol 70:282–291
Perng GC, Ghiasi H, Slanina SM, Nesburn AB, Wechsler SL (1996b) The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3- kilobase primary transcript. J Virol 70:976–984
Perng GC, Slanina SM, Ghiasi H, Nesburn AB, Wechsler SL (1996c) A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J Virol 70:2014–2018
Perng GC, Ghiasi H, Slanina SM, Nesburn AB, Wechsler SL (1996d) High-dose ocular infection with a herpes simplex virus type 1 ICP34.5 deletion mutant produces no corneal disease or neurovirulence yet results in wild-type levels of spontaneous reactivation. J Virol 70:2883–2893
Perng G, Jones C, Ciacci-Zanella H, Henderson G, Yukht A, Slanina S, Hofman F, Ghiasi H, Nesburn A, Wechsler S (2000) Virus induced neuronal apoptosis blocked by the herpes simplex virus latency associated transcript (LAT). Science 287:1500–1503
Perng GC, Esmaili D, Slanina SM, Yukht A, Ghiasi H, Osorio N, Mott KR, Maguen B, Jin L, Nesburn AB, Wechsler SL (2001a) Three herpes simplex virus type 1 latency-associated transcript mutants with distinct and asymmetric effects on virulence in mice compared with rabbits. J Virol 75:9018–9028
Perng GC, Slanina SM, Ghiasi H, Nesburn AB, Wechsler SL (2001b) The effect of latency-associated transcript on the herpes simplex virus type 1 latency-reactivation phenotype is mouse strain-dependent. J Gen Virol 82:1117–1122
Perng GC, Maguen B, Jin L, Mott KR, Osorio N, Slanina SM, Yukht A, Ghiasi H, Nesburn AB, Inman M, Henderson G, Jones C, Wechsler SL (2002a) A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol 76:1224–1235
Perng GC, Mott KR, Osorio N, Yukht A, Salina S, Nguyen QH, Nesburn AB, Wechsler SL (2002b) Herpes simplex virus type 1 mutants containing the KOS strain ICP34.5 gene in place of the McKrae ICP34.5 gene have McKrae-like spontaneous reactivation but non-McKrae-like virulence. J Gen Virol 83:2933–2942
Rock DL, Nesburn AB, Ghiasi H, Ong J, Lewis TL, Lokensgard JR, Wechsler SL (1987) Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol 61:3820–3826
Rock D, Lokensgard J, Lewis T, Kutish G (1992) Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J Virol 66:2484–2490
Sacks WR, Schaffer PA (1987) Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol 61:829–839
Samoto K, Ehtesham M, Perng GC, Hashizume K, Wechsler SL, Nesburn AB, Black KL, Yu JS (2002) A herpes simplex virus type 1 mutant with gamma 34.5 and LAT deletions effectively oncolyses human U87 glioblastomas in nude mice. Neurosurgery 50:599–605, discussion 605-596
Sinani D, Jones C (2011) Localization of sequences in a protein (ORF2) encoded by the latency-related gene of bovine herpesvirus 1 that inhibits apoptosis and interferes with Notch1-mediated trans-activation of the bICP0 promoter. J Virol 85:12124–12133, PMC:3209353
Smith RE, McDonald HR, Nesburn AB, Minckler DS (1980) Penetrating keratoplasty: changing indications, 1947 to 1978. Arch Ophthalmol 98:1226–1229
Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT (1987) RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056–1059
Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR (2008) MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–783
Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR (2009) Analysis of human alphaherpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol 83:10677–10683
Whitley RJ (1997) Herpes simplex virus. In: Scheld W, Whitley RJ, Durack D (eds) Infections of the central nervous system, 2nd edn. Lippincott-Raven, Philadelphia, pp 73–89
Acknowledgments
This study was supported by Public Health Service NIH grants R01EY013191, 1R56AI098985, 1R56AI093133, RO1EY019896, and RO1EY14900, and The Discovery Center for Eye Research. We thank Dr. Nigel Fraser for reading this manuscript and providing helpful comments.
Conflict of interest
All of the authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, X., Brown, D., Osorio, N. et al. A herpes simplex virus type 1 mutant disrupted for microRNA H2 with increased neurovirulence and rate of reactivation. J. Neurovirol. 21, 199–209 (2015). https://doi.org/10.1007/s13365-015-0319-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-015-0319-1