Abstract
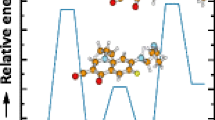
A new hexadentate, tripodal 8-hydroxyquinoline based ligand (QH3) and its gadolinium(III) tris-chelated (GdQ) complex with hemicage structure was investigated by using high resolution Fourier-transform ion cyclotron resonance mass spectrometry (FTICRMS). The protonated adduct of the free ligand and its hemicage tripodal Gd(III) complex, [GdQ + H]+, were first observed in experiments of electrospray ionization (ESI) with a linear ion trap (LTQ) mass spectrometer and further investigated by using high resolution FTICRMS. Gas-phase dissociation of the protonated Gd(III) complex, by infrared multiphoton dissociation (IRMPD) FTICR MS, demonstrated a fragmentation pattern with six main product cluster ions labeled as [Fn]+ (n = 1 up to 6). These product ions suggest the elimination of 7-amino-alkyl or 7-alkyl chains of the hemicage moiety. High resolution MS conditions allowed the elucidation of the fragmentation pattern and product ion structures along with the determination, among the isotopic pattern of Gd, of the chemical compositions of closely related species, which differ in terms of hydrogen content. Among the Gd six naturally stable isotopes, 158Gd is the most abundant, and its peak within each cluster was used as a reference for distinguishing each product ions. Computational DFT investigations were applied to give support to some hypothesis of fragmentation pathways, which could not have been easily justified on the basis of the experimental work. Furthermore, computational studies suggested the coordination geometry of the protonated parent complex and the five- and four-coordinated complexes, which derive from its fragmentation. Furthermore, experimental and computational evidences were collected about the octet spin state of the parent compound.









Similar content being viewed by others
References
Santos, L.S. (ed.): Reactive Intermediates: MS Investigations in Solution. Wiley-VCH, Weinheim (2010)
Dyson, P.J., Scott McIndoe, J.: Analysis of organometallic compounds using ion trap mass spectrometry. Inorg. Chim. Acta 354, 68–74 (2003)
Qian, R., Guo, H., Liao, Y., Guo, Y., Ma, S.: Probing the mechanism of palladium-catalyzed addition of organoboronic acids to allenes in the presence of AcOH with ESI-FTMS. Angew. Chem. Int. Ed. 44, 4771–4774 (2005)
Mosely, J.A., Murray, B.S., Parker, D.: Electron-capture dissociation and collision-induced dissociation of lanthanide metal-ligand complexes and lanthanide metal-ligand complexes bound to phosphopeptides. Eur. J. Mass Spectrom. 15(2), 145–155 (2009)
Lau, R.L.C., Jiang, J., Ng, D.K.P., Chan, T.-W.D.: Fourier Transform ion cyclotron resonance studies of Lanthanide(III) porphyrin-phthalocyanineheteroleptic sandwich complexes using electrospray ionization. J. Am. Soc. Mass Spectrom. 8, 161–169 (1997)
Godin, B., Tasciotti, E., Liu, X., Serda, R.E., Ferrari, M.: Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc. Chem. Res. 18, 979–989 (2011)
Henig, J., Tóth, E., Engelmann, J., Gottschalk, S., Mayer, H.A.: Macrocyclic Gd3+ chelates attached to a silsesquioxane core as potential magnetic resonance imaging contrast agents: synthesis, physicochemical characterization, and stability studies. Inorg. Chem. 49, 6124–6138 (2010)
Soroka, K., Vithanage, R.S., Phillips, D.A., Walker, B., Dasgupta, P.K.: Fluorescence properties of metal complexes of 8-hydroxyquinoline-5-sulfonic acid and chromatographic applications. Anal. Chem. 59, 629–636 (1987)
Hollingshead, R.G.W.: Oxine and Its Derivatives, vol. I-IV. Butterworths, London (1954–1956)
Phillips, J.P.: The reactions of 8-quinolinol. Chem. Rev. 56, 271–297 (1956)
Hollingshead, R.G.W.: Studies on oxine and its derivatives: the sensitivity and selectivity of some 7-substituted 8-hydroxyquinoline-5-sulphonic acids and some 2-substituted 8-hydroxyquinoline-4-carboxylic acids towards certain metals. Anal. Chim. Acta 19, 447–457 (1958)
Dowlings, S.D., Seitz, W.R.: Effect of metal–ligand ratio on polarization of fluorescence from metal-8-quinolinol complexes. Spectrochim. Acta Part A: Mol. Spectrosc. 40, 991–993 (1984)
Fernandez-Gutierrez, A., Munoz de la Pena, A.: In: Schulman, S.G. (ed.) Molecular luminescence spectroscopy. Methods and Application, Part I, pp. 371–546. Wiley, New York (1985)
Farruggia, G., Iotti, S., Prodi, L., Montalti, M., Zaccheroni, N., Savage, P.B., Trapani, V., Sale, P., Wolf, F.I.: 8-hydroxyquinoline derivatives as fluorescent sensors for magnesium in living cells. J. Am. Chem. Soc. 128, 344–350 (2006)
Bardez, E., Devol, I., Larrey, B., Valeur, B.: Excited State Processes in 8-hydroxyquinoline: photoinduced tautomerization and solvation effects. J. Phys. Chem. B 101, 7786–7793 (1997)
Amati, M., Belviso, S., Cristinziano, P., Minichino, C., Lelj, F., Aiello, I., La Deda, M., Ghedini, M.: 8-Hydroxyquinoline monomer, water adducts, and dimer. Environmental influences on structure, spectroscopic properties, and relative stability of cis and trans conformers. J. Phys. Chem. 111, 13403–13414 (2007)
Tang, C.W., VanSlyke, S.A.: Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913–915 (1987)
Zhigang, L., Hong, M.: Organic Light-EmittingMaterials and Devices, Chap. 3. Boca Raton, CRC Press: Taylor and Francis Group (2007)
Amati, M., Lelj, F.: Luminescent compounds fac- and mer-aluminum tris(quinolin-8-olate). A pure and hybrid density functional theory and time-dependent density functional theory investigation of their electronic and spectroscopic properties. J. Phys. Chem. A 107, 2560–2569 (2003)
Tallec, G., Imbert, D., Fries, P.H., Mazzanti, M.: Highly stable and soluble bis-aqua Gd, Nd. Yb complexes as potential bimodal MRI/NIR Imaging Agents. Dalton Trans. 39, 9490–9492 (2010)
Eliseevaa, S.V., Bünzli, J.-C.G.: Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 39, 189–227 (2010)
Marshall, A.G., Hendrickson, C.L., Jackson, G.S.: Fourier transform ion cyclotron resonance mass spectrometry: a primer. Mass Spectrom. Rev. 17, 1–35 (1998)
Marshall, A.G.: Milestones in Fourier transform ion cyclotron resonance mass spectrometry technique development. Int. J. Mass Spectrom. 200, 331–356 (2000)
Gauthier, J.W., Trautman, T.R., Jacobson, D.B.: Sustained off-resonance irradiation for collision-activated dissociation involving Fourier transform mass spectrometry. Collision-activated dissociation technique that emulates infrared multiphoton dissociation. Anal. Chim. Acta 246, 211–225 (1991)
Woodlin, R.L., Bomse, D.S., Beauchamp, J.L.: Multiphoton dissociation of molecules with low power continuous wave infrared laser radiation. J. Am. Chem. Soc. 100, 3248–3250 (1978)
Little, D.P., Speir, J.P., Senko, M.W., O’Connor, P.B., McLafferty, F.W.: Infrared multiphoton dissociation of large multiply-charged ions for biomolecule sequencing. Anal. Chem. 66, 2809–2815 (1994)
Dunbar, R.C., McMahon, T.B.: Activation of unimolecular reactions by ambient blackbody radiation. Science 279, 194–197 (1998)
Price, W.D., Schnier, P.D., Williams, E.R.: Tandem mass spectrometry of large biomolecule ions by blackbody infrared radiative dissociation. Anal. Chem. 68, 859–866 (1996)
McCormack, A.L., Jones, J.L., Wysocki, V.H.: Surface-induced dissociation of multiply protonated peptides. J. Am. Soc. Mass Spectrom. 3, 859–862 (1992)
Chorush, R.A., Little, D.P., Beu, S.C., Wood, T.D., McLafferty, F.W.: Surface induced dissociation of multiply protonated proteins. Anal. Chem. 67, 1042–1046 (1995)
Cooper, H.J., Hakansson, K., Marshall, A.G.: The role of electron capture dissociation in biomolecular analysis. Mass Spectrom. Rev. 24, 201–222 (2005)
Chrisman, P.A., Pitteri, S.J., McLuckey, S.A.: Parallel ion parking: improving conversion of parents to first-generation products in electron transfer dissociation. Anal. Chem. 77, 3411–3414 (2005)
Sleno, L., Volmer, D.A.: Ion activation methods for tandem mass spectrometry. J. Mass Spectrom. 39, 1091–1112 (2004)
Laskin, J., Futrell, J.H.: Activation of large ions in FT-ICR mass spectrometry. Mass Spectrom. Rev. 24, 135–167 (2005)
Cooper, H.J., March, R.E., Todd, J.F.J.: Practical aspects of ion trap mass spectrometry. Vol. V, Applications to ion trapping devices. CRC Press, Boca Raton (2010)
McFarland, M.A., Marshall, A.G., Hendrickson, C.L., Nilsson, C.L.: Structural characterization of the gm1 ganglioside by infrared multiphoton dissociation, electron capture dissociation, and electron detachment dissociation electrospray ionization FT-ICR MS/MS. J. Am. Soc. Mass Spectrom. 16, 752–762 (2005)
Polfer, N.C.: Infrared multiple photon dissociation spectroscopy of trapped ions. Chem. Soc. Rev. 40, 2211–2221 (2011)
Bianco, G., Labella, C., Pepe, A., Cataldi, T.R.I.: Scrambling of autoinducing precursor peptides investigated by infrared multiphoton dissociation with electrospray ionization and Fourier-transform ion cyclotron resonance mass spectrometry. Anal. Bioanal. Chem. 405(5), 1721–1732 (2013)
Cataldi, T.R.I., Ricciardi, G., Bianco, G., Pietrangeli, D., Abate, S.: Mass spectrometric evidence for collisionally induced removal of H(2) from monoanions of (10)B nido-carborane derivatives investigated by electrospray ionization quadrupole linear ion trap and Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 23, 1927–1933 (2009)
Zhao, Y., Truhlar, D.G.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor. Chem. Acc. 120, 215–241 (2008)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, N.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.1. Gaussian, Inc, Wallingford CT (2009)
Ditchfield, R., Hehre, W.J., Pople, J.A.: Self-consistent molecular orbital methods. 9. Extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1971)
Hehre, W.J., Ditchfield, R., Pople, J.A.: Self-consistent molecular orbital methods. 1. Use of Gaussian expansions of Slater-type atomic orbitals. J. Chem. Phys. 56, 2257–2261 (1972)
Hariharan, P.C., Pople, J.A.: Accuracy of AH equilibrium geometries by single determinant molecular-orbital theory. Mol. Phys. 27, 209–214 (1974)
Gordon, M.S.: The isomers of silacyclopropane. Chem. Phys. Lett. 76, 163–168 (1980)
Hariharan, P.C., Pople, J.A.: Influence of polarization functions on molecular-orbital hydrogenation energies. Theo. Chim. Acta 28, 213–222 (1973)
Nicklass, A., Dolg, M., Stoll, H., Preuss, H.: Ab initio energy-adjusted pseudopotentials for the noble gases Ne through Xe: calculation of atomic dipole and quadrupole polarizabilities. J. Chem. Phys. 102, 8942–8952 (1995)
Bacskay, G.B.A.: Quadratically convergent Hartree-Fock (QC-SCF) method. Application to closed systems. Chem. Phys. 61, 385–404 (1981)
Fluckiger, P., Luthi, H.P., Portmann, S., Weber, J.: MOLEKEL 4.3. Swiss Center for Scientific Computing, Manno (2000–2002)
Portmann, S., Luthi, H.P.: MOLEKEL: an interactive molecular graphic tool. Chimia 54, 766–770 (2000)
De Bonis, M., Lelj, F. et al. manuscript in preparation.
Wang, J., Oyler, K.D., Bernhard, S.: Synthesis and characterization of hemicaged 8-hydroxyquinoline chelates with enhanced electrochemical and photophysical properties. Inorg. Chem. 46, 5700–5706 (2007)
Evans, D. F.: Determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 2003–2005 (1959). doi:10.1039/JR9590002003
Dickinson, W.C.: The time average magnetic field at the nucleus in nuclear magnetic resonance experiments. Phys. Rev. 81, 717–731 (1951)
Bain, A.G., Berry, J.F.: Diamagnetic Corrections and Pascal’s Constants. J. Chem. Ed. 85, 532–535 (2008)
Weber, R.J.M., Southam, A.D., Sommer, U., Viant, M.R.: Characterization of isotopic abundance measurements in high resolution FT-ICR and Orbitrap mass spectra for improved confidence of metabolite identification. Anal. Chem. 83, 3737–3743 (2011)
do Lago, C.L., Kascheres, C.: New method of isotope pattern analysis. Comput. Chem. 15, 149–155 (1991)
Rosman, K.J.R., Taylor, P.D.P.: Isotopic compositions of the elements 1997 (Technical Report). Pure Appl. Chem. 70, 217–235 (1998)
Henderson, W., Scott McIndoe, J.S.: The ESI MS behavior of main group organometallic compounds. Mass spectrometry of inorganic, coordination, and organometallic compounds, Chap 6. John Wiley, Chichester (2005)
Audi, G., Wapstra, A.H.: The 1995 update to the atomic mass evaluation. Nucl. Phys. A 595, 409–480 (1995)
Purcell, K. F., Kotz, J. C. In: Inorganic Chemistry, Chap 10. International Edition Holt-Saunders, Japan (1985)
Sun, L.-N., Zhang, H.-J., Yu, J.-B., Yu, S.-Y., Peng, C.-Y., Dang, S., Guo, X.-M., Feng, J.: Near-Infrared emission from novel tris(8-hydroxyquinolinate)lanthanide(III) Complexes-functionalized Mesoporous SBA-15. Langmuir 24, 5500–5507 (2008)
Bertini, I., Luchinat, C.: NMR of paramagnetic substances. Coord. Chem. Rev. 150, 1–300 (1996)
Acknowledgment
This work was performed by using the instrumental facilities of CIGAS Center supported by EU (Project no. 2915/12), Regione Basilicata and Università degli Studi della Basilicata (USB). M.D.B. thanks USB for the university research fellowship, which allowed her to work in the field of 8-hydroxyquinoline hemicage ligands for metal complexation. This work was supported by Consortium INSTM through the PRISMA 2007 project.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 833 kb)
Rights and permissions
About this article
Cite this article
De Bonis, M., Bianco, G., Amati, M. et al. An Interplay Between Infrared Multiphoton Dissociation Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry and Density Functional Theory Computations in the Characterization of a Tripodal Quinolin-8-Olate Gd(III) Complex. J. Am. Soc. Mass Spectrom. 24, 589–601 (2013). https://doi.org/10.1007/s13361-012-0570-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-012-0570-0