Abstract
A strategy based on negative ion electrospray ionization tandem mass spectrometry and closed-ring labeling with both 8-aminopyrene-1,3,6-trisulfonate (APTS) and p-aminobenzoic acid ethyl ester (ABEE) was developed for linkage and branch determination of high-mannose oligosaccharides. X-type cross-ring fragment ions obtained from APTS-labeled oligosaccharides by charge remote fragmentation provided information on linkages near the non-reducing terminus. In contrast, A-type cross-ring fragment ions observed from ABEE-labeled oligosaccharides yielded information on linkages near the reducing terminus. This complementary information provided by APTS- and ABEE-labeled oligosaccharides was utilized to delineate the structures of the high-mannose oligosaccharides. As a demonstration of this approach, the linkages and branches of high-mannose oligosaccharides Man5GlcNAc2, Man6GlcNAc2, Man8GlcNAc2, and Man9GlcNAc2 cleaved from the ribonuclease B were assigned from MS2 spectra of ABEE- and APTS-labeled derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glycosylation is a predominant post-translational modification as 50 % of mammalian proteins are glycosylated [1–6]. The structural diversity inherent in the sugar moieties of a glycoprotein results in subtle changes in protein shape and charge, ultimately affecting protein function, both temporally and spatially.
Mass spectrometry is one of the most powerful and versatile tools for structural analysis of carbohydrates [7]. Soft ionization techniques such as matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) are now the most popular ionization methods used in carbohydrate structural analysis [8–38].
Metal ion coordination [13–15], permethylation [16–20, 31–38], reducing-end labeling [21–30], or underivatized glycans [9–12] in combination with tandem mass spectrometry are among reported strategies to produce linkage and branch information. Among these approaches, analysis of permethylated glycans by MALDI-TOF/TOF is quite useful [31–38]. Unfortunately, it requires the use of high-energy collision-induced dissociation (he-CID), which is not available in many laboratories studying oligosaccharides.
Negative ion spectra of underivatized oligosaccharides could produce many linkage specific fragment ions. However, for oligosaccharides larger than a hexasaccharide, the use of MS3 or MS4 is needed. In addition, linkage information on non-reducing end and the linkage information on the 3-linked branch are prone to miss [9–12]. The fragmentation patterns of closed-ring labeled and underivatized oligosaccharides are very similar [9–12, 22]. However, our earlier study of ABEE-labeled oligosaccharides by negative ion ESI showed that MS sensitivity was enhanced and a more clear assignment for the linkages was obtained [22, 28]. Unfortunately, for oligosaccharides larger than a hexasaccharide, linkages near the non-reducing end were not observed. To extend this ABEE approach to larger oligosaccharides, a procedure involving alkaline degradation was introduced prior to labeling and MSn analysis. Consequently, linkage and branch information near the non-reducing end were obtained [29].
To provide linkage information near the non-reducing end of oligosaccharide, fragmentation near the non-reducing end is required. Trimethyl(p-aminophenyl)ammonium (TMAPA), a fixed-charged derivative, has been studied as a labeling reagent for oligosaccharide analysis [39]. Fragmentation is observed exclusively at the non-reducing terminus with charge retention on the TMAPA label at the reducing terminus. Based on this concept, oligosaccharides were separately labeled with both ABEE and the fixed-charge derivative, 8-aminopyrene-1,3,6-trisulfonate (APTS), before negative ESI-MS/MS analysis. The complementary information provided by ABEE- and APTS-labeled oligosaccharides was utilized to elucidate the structure of oligosaccharides larger than a hexasaccharide. The potential of this combined approach was demonstrated using the high-mannose glycans cleaved from ribonuclease B.
2 Experimental
2.1 Materials
p-Aminobenzoic acid ethyl ester, maltohexaose, kojibiose, and ribonuclease B were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). 8-Aminopyrene-1,3,6-trisulfonate was purchased from Fluka (Buchs, Switzerland). Laminarihexaose and isomaltohexaose were purchased from Seikagaku Kogyo (Tokyo, Japan). Peptide-N-glycanase F (PNGase F) was purchased from Roche Biochemicals (Basel, Switzerland). Glacial acetic acid, methanol, and acetonitrile were purchased from J. T. Baker (Phillipsburg, NJ, USA). Deionized (18MΩ) water (Milli-Q water system, Millipore Inc., Bedford, MA, USA) was used in the preparation of the samples and reaction solution.
2.2 Preparation of N-Linked Glycans
The N-linked glycans were released enzymatically from ribonuclease B by means of PNGase F digestion and purified by using graphitized carbon cartridges (Alltech Associates, Lancashire, UK) [40, 41] and evaporated to dryness.
2.3 Preparation of Closed-Ring Chromophore Labeled Derivatives
For APTS labeling, dried oligosaccharides were derivatized by the addition of 2 μL 0.02 M APTS in 25 % acetic acid and 10 μL deionized water in an Eppendorf tube. The solution was kept at 75 °C for 6 h. The derivatives were purified by passing through a column made of Sephadex G-25 (Sigma) using water as the eluent, followed by lyophilization. For ABEE labeling, dried glycans were derivatized by using the glycosylamine approach [29].
2.4 Electrospray Mass Spectrometry
All mass spectrometry experiments were performed using a Finnigan LTQ ion trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA) under negative ion electrospray. Samples were infused by syringe pump at a rate of 200 nL/min. The heated capillary was maintained at 275 °C for all experiments. For mass-analyzer collision-induced dissociation (CID), relative collision energy of 35 %–50 % was used in MS2 experiments.
3 Results and Discussion
3.1 The Linkage Analysis of APTS-Labeled Oligosaccharides
A typical negative ESI mass spectrum of APTS-labeled oligosaccharide (spectrum not shown) is characterized by the doubly and triply charged molecular ions along with the sodiated adducts. The [M – 3 H + Na]2– ion was chosen as the precursor ion because it produced more linkage related cross-ring cleavage fragments when compared to MS2 analysis of the [M – 2 H]2– and [M – 3 H]3– ions (data not shown).
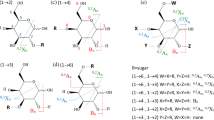
Kojibiose, laminarihexaose, maltohexaose, and isomaltohexaose are 1-2, 1-3, 1-4, and 1-6 linked linear oligosaccharides, respectively. They were closed-ring labeled with APTS and studied by negative ion ESI-MS2 (Figures 1 and 2). The results revealed that the fragmentation patterns of APTS-labeled oligosaccharides occurred primarily via charge-remote fragmentation. As a result, linkage information starting at the non-reducing end was provided. A summary of the observed linkage specific fragment ions is shown in Table 1. Owing to the lack of a standard containing an internal 1-2 linkage, ions specific for internal 1-2 linkages were not assigned.
MS2 negative ESI mass spectra of APTS-labeled oligosaccharides. (a) MS2 spectrum of APTS-labeled kojibiose ([M – 3H + Na]2–, m/z 400.5). (b) MS2 spectrum of APTS-labeled laminarihexaose ([M – 3H + Na]2–, m/z 724.5). The specific fragment ions were assigned based on the nomenclature proposed by Domon and Costello [43]
3.2 Analysis of Man5GlcNAc2 Based on ABEE and APTS-Labeling
For oligosaccharides larger than a hexasaccharide, such as Man5GlcNAc2, the linkages near the reducing-end could not be determined based on the spectrum of APTS labeling (Figure 3b). A strategy incorporating both APTS and ABEE labeling was employed to assign all linkages of Man5GlcNAc2.
The MS2 spectrum of ABEE-labeled Man5GlcNAc2 is shown in Figure 3a. It is noted that the linkage assignment of ABEE-labeled Man5GlcNAc2 has previously been reported [29]. The fragment ions 0,2A5-18 (m/z 1114.2), 0,2A4 (m/z 929.2), and 0,2A4-18 (m/z 911.2) indicated that the first two linkages were 1-4 linkages. The mass difference between C3 (m/z 827.1) and C3/Z3β-H (m/z 647.1) ion represents a 3-linked Hex connected to the first branch. The fragment ions C3/Z3β-H-18 (m/z 629.2), 0,3A3 (m/z 575.1), and 0,4A3 (m/z 545.3) revealed that the first branch has a 3-,6-linked sugar.
The linkages near the non-reducing terminal were deduced from the MS2 spectrum of APTS-labeled Man5GlcNAc2 (Figure 3b). The fragment ions at m/z 816.6 (0,4X4α'), m/z 795.6 (2,5X4α') and m/z 786.6 (2,5X4α' – H2O) suggested that one of the terminal mannose moieties binds by a 1-6 glycosyl bond. In addition, two series of terminal 1-3 linked characteristic ions were observed. The ions at m/z 735.2 (2,4X4'' /Y4α') and m/z 720.6 (0,3X4'' /Y4') represent a 1-3 linked terminal mannose with the loss of a monosaccharide. The ions at m/z 574.6 (2,4X3β/Y3α) and m/z 560.5 (0,3X3β /Y3α) represent a 1-3 linked terminal mannose with the loss of a trisaccharide. The C2α ion (m/z 503.1) in Figure 3a and Z 3α ion (m/z 595.1) in Figure 3b suggested that a trisaccharide is connected to the 6-arm of the first branch. Therefore, these two ions were assigned as the cross-ring cleavage ions from the 3-linked mannose connected to the first branch. The 1-6 linked terminal and the second 1-3 linked terminal sugar were assigned as connected to the 6-arm of the first branch. As a result, a second branch point connected to the 6-arm of the first branched sugar was assigned as a 1-3 and 1-6 linked mannose.
3.3 Analysis of Man6GlcNAc2 Based on ABEE and APTS-Labeling
The MS2 spectrum of ABEE-labeled Man6GlcNAc2 is shown in Figure 4a. The assignment is similar to Man5GlcNAc2, except that the mass difference between C3 (m/z 989.2) and C3/Z3β – H (m/z 647.1) ion (342 Da) suggested that a disaccharide was connected to the 3-branch.
The MS2 spectrum of APTS-labeled Man6GlcNAc2 is shown in Figure 4b. In addition to the terminal 1-3 and 1-6 linked mannoses as in Man5GlcNAc2, a 1-2 linked terminal mannose was assigned by the characteristic ions 0,2X4β/Y3α (m/z 624.3) and 0,2X4β – H2O/Y3α (m/z 614.8). The characteristic ion 2,5X3α (m/z 715.4) suggested that the second branch did not contain a 1-2 linked mannose, therefore, the 2-linked terminal mannose was assigned as the terminal sugar of the 3-linked disaccharide in the first branch. The observed 1-3 linked internal fragments 1,4X3β/Y3α (m/z 560.7) was assigned as the 3-linked disaccharide connected to the first branch. Other assignments were similar to Man5GlcNAc2.
3.4 Analysis of Man8GlcNAc2 Based on APTS and ABEE-Labeling
The Man8GlcNAc2 is a mixture of three structural isomers with a molar ratio of ~ 84 : 6 : 10 [42]. Because of the much higher abundance of one isomer (84 %), the fragmentation is expected to be predominated by this isomer.
The MS2 spectrum of ABEE-labeled Man8GlcNAc2 (Supplemental Figure S1) is similar to Man6GlcNAc2 (Figure 4a). The mass difference between C4 (m/z 1313.2) and C4/Z3β – H (m/z 809.2) ion (504 Da) suggested that the 3-arm was substituted with a trisaccharide. In addition, the fragment ion C3α (m/z 665.2) indicated that a tetrasaccharide was connected to the 6-arm. Other assignments were the same as in Man5GlcNAc2, and the predicted structure is shown in Figure 5a-1.
The MS2 spectrum of APTS-labeled Man8GlcNAc2 is shown in Figure 5b. Three terminal (two 1-2, one 1-3 linked) and three internal mannoses (1-3, 1-6, 1-2 linked) were observed (Supplemental Table S1). The characteristic ion 1,4X3β/Y3α(m/z 560.5) corresponds to an internal 1-3 linked mannose with the loss of the 6-arm of the first branch. It suggested that a trisaccharide was connected to the first branch through a 1-3 linkage. This assignment is consistent with the assignment based on the C4/Z3β – H ion (m/z 809.2) observed in ABEE derivative. The ions at m/z 705.6 (0,2X5β/Y3α) and m/z 696.6 (0,2X5β – H2O/Y3α) correspond to a terminal 1-2 linked mannose with a loss of the 6-arm. It suggested that the terminal linkage of the 3-linked trisaccharide was a 1-2 linkage (Figure 5a-2). The ions at m/z 897.6 (2,4X4α"/ Y4') and m/z 883.4 (0,3X4α"/ Y4') correspond to a terminal 1-3 mannose with loss of a disaccharide. It suggests that Man8GlcNAc2 contained three terminal mannoses (Figure 5a-3). The fragment ions 0,2X5α'or 5β (m/z 1029.7) and 0,2X5α' or 5β-H2O (m/z 1020.7) resulted from a terminal 1-2 linked mannose suggested that the terminal linkage of the 6-arm was a 1-2 linkage (Figure 5a-4).
The fragment ion 1,4X3β/Y3α suggested that position C6 of the 3-linked internal mannose was not substituted (otherwise, a fragment ion would appear two hexoses higher than m/z 560.5). Therefore, the internal 1-6 linked mannose was assigned to the second branched sugar (Figure 5a-5). The second linkage of 3-arm was assigned as an internal 1-2 linkage (Figure 5a-6). Because of the lack of a standard with internal 1-2 linkage, the assignment was based on two sources of indirect evidence. The 1-6 linkage was ruled out as described in the first sentence of this paragraph. Due to the absence of fragments derived from either internal 1-4 or 1-3 linked mannose on the 3-arm, the second linkage was assigned as a 1-2 linkage. This assignment was also supported by observation of the 1,3X4β – H2O/Y3α (m/z 646.7) and 1,5X4β – H2O/Y3α (m/z 610.2) ions, which differ from the characteristic ions of internal 1-3, 1-4, and 1-6 linkages and were tentatively assigned as ions specific to internal 1-2 linkage. As a result, the linkages and branches of Man8GlcNAc2 were assigned.
In this analysis of Man8GlcNAc2, it is assumed that the mass spectrum was dominated by the most abundant isomer (84 %). However, it should be cautious with this assumption, because many characteristic ions are of low abundance and the mass spectrum is normalized out of one dimensional plot.
3.5 Analysis of Man9GlcNAc2 Based on APTS and ABEE-Labeling
The fragment ion C3α (m/z 827.1) in MS2 spectrum of ABEE-labeled Man9GlcNAc2 (Supplemental Figure S2) suggested that a pentasaccharide was connected to the 6-arm of the first branch. Other assignments were the same as in Man8GlcNAc2, and the predicted structure is shown in Figure 6a-1.
The MS2 spectrum of APTS-labeled Man9GlcNAc2 is shown in Figure 6b. Three terminal 1-2 linked and four internal mannoses (one 1-6, one 1-2, and two 1-3) were observed (Supplemental Table S2). The characteristic ion 1,4X4α'/Y4α'' (m/z 883.5) corresponds to an internal 1-3 linked mannose (second from the terminus) with the loss of a disaccharide. This ion suggested that the 6-arm of the first branched sugar had a second branched sugar, and that a disaccharide was linked to the second branch through a 1-3 linkage (Figure 6a-2). The ions at m/z 705.6 (0,2X5β/Y3α) and 696.6 (0,2X5β – H2O/Y3α) were produced from a terminal 1-2 linked mannose with a loss of a pentasaccharide from the 6-arm. It indicated that the terminal linkage of the 3-linked trisaccharide was a 1-2 linkage (Figure 6a-3). The fragment ions 0,2X5α'/Y4α'' (m/z 949.2) and 0,2X5α' – H2O/Y4α'' (m/z 940.4) correspond to a terminal 1-2 linked mannose with the loss of a disaccharide. The fragment ions 0,2X5α'or 5β (m/z 1110.7) and 0,2X5α' or 5β –H2O (m/z 1102.1) correspond to a terminal 1-2 linked mannose with no loss of the sugar. These two series of terminal 1-2 linked fragment ions suggested that the two termini of the second branched sugar were 1-2 linked (Figure 6a-4). The determination of two unassigned linkages in Figure 6b-4 was the same as in Man8GlcNAc2. As a result, the linkages and branches were assigned (Figure 6a-5)
3.6 Analysis of Man7GlcNAc2 Isomers
The Man7GlcNAc2 is a mixture of three isomers with similar abundance. The structures of the isomers and the MS2 spectrum of APTS-labeled Man7GlcNAc2 were shown in Supplemental Figure S3. Many product ions were observed and some linkage specific fragments suggested that more than one isomer was present in the sample analyzed. For example, the fragments at m/z 898.2 (2,4X4α''/Y4α'), m/z 883.1(0,3X4α''/Y4α') and at m/z 816.8 (2,4X4α''/Y4α'), m/z 801.8 (0,3X4α''/Y4α') correspond to a terminal 1-3 linked mannose with the loss of a monosaccharide (isomer I) and a disaccharide (isomer III) from the 6-arm, respectively.
The linkage specific fragments were assigned and summarized in Supplemental Table S3. As can be seen, linkage specific fragments related to the three isomers were observed. However, it should be mentioned that without knowing the structure of the isomers, some fragments could be assigned to different structures. For example, fragments labeled with (X) could be assigned as the linkage specific fragments with an assumptive structure of X. As a result, when this approach is used to analyze an unknown sample, purification before MS2 analysis is highly recommended.
4 Conclusions
Negative-ion ESI-MS/MS analysis of APTS closed-ring labeled oligosaccharides showed that linkages can be assigned for oligosaccharides up to six sugar residues. Unlike the ABEE labeling, the linkage information is mainly derived from the non-reducing end. This complementary information produced by ABEE- and APTS-labeled oligosaccharides was utilized to delineate the linkages and branches of larger oligosaccharides. The analysis of N-linked glycans from ribonuclease B showed that the linkages and branches for Man5GlcNAc2, Man6GlcNAc2 Man8GlcNAc2 and Man9GlcNAc2 could be deduced from the combined information obtained from MS2 of ABEE- and APTS-labeled oligosaccharides. It is worth to mention that low-energy MS2 instead of high-energy MS2 or MSn is used in this study. As a result, this approach can be adopted by laboratories equipped with triple quadrupole, quadrupole time-of-flight (Q/TOF) or ion trap mass spectrometer. High-mannose glycans studied in this approach are well characterized oligosaccharides. There could be many other structural isomers. Isomers not examined in this approach may present identical or very similar mass spectra. Therefore, the assignments may not be fully conclusive when this approach is applied to true unknown.
References
Li, H., Sharon, N.: Protein glycosylation: Structural and functional aspects. Eur. J. Biochem. 218, 1–27 (1993)
Dwek, R.A.: Glycobiology: Toward understanding the function of sugars. Chem. Rev. 96, 683–720 (1996)
Kukuruzinska, M.A., Lennon, K.: Protein N-glycosylation: Molecular genetics and functional significance. Crit. Rev. Oral Biol. Med. 9, 415–448 (1998)
Bernfield, M., Gotte, M., Park, P.W., Reizes, O., Fitzgerald, M.L., Lincecum, J., Zako, M.: Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999)
Sugahara, K., Kitagawa, H.: Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 10, 518–527 (2000)
Helenius, A., Aebi, M.: Intracellular functions of N-linked glycans. Science 291, 2364–2369 (2001)
Chaplin, M.F., Kennedy, J.F.: Carbohydrate Analysis, pp. 221–289. Oxford Univ. Press, New York (1994)
Tseng, K., Hedrick, J.L., Lebrilla, C.B.: Catalog-library approach for the rapid and sensitive structural elucidation of oligosaccharides. Anal. Chem. 71, 3747–3754 (1999)
Chai, W., Lawson, A.M., Piskarev, V.: Branching pattern and sequence analysis of underivatized oligosaccharides by combined MS/MS of singly and doubly charged molecular ions in negative-ion electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 670–679 (2002)
Pfenninger, A., Karas, M., Finke, B., Stahl, B.: Structural analysis of underivatized natural human milk oligosaccharides in the native ion mode by nano-electrospray MSn. Part 1: Methodology. J. Am. Soc. Mass Spectrom. 13, 1331–1340 (2002)
Morelle, W., Michalski, J.-C.: The mass spectrometric analysis of glycoproteins and their glycan structures. Curr. Anal. Chem. 1, 29–57 (2005)
Zhang, Z., Linhardt, R.J.: Sequence analysis of native oligosaccharides using negative ESI tandem MS. Curr. Anal. Chem. 5, 225–237 (2009)
Sible, E.M., Brimmer, S.P., Leary, J.A.: Interaction of first row transition metals with a 1-3, a 1-6 mannotriose, and conserved trimannosyl core oligosaccharides: A comparative electrospray ionization study of doubly and singly charged complexes. J. Am. Soc. Mass Spectrom. 8, 32–42 (1997)
König, S., Leary, J.A.: Evidence for linkage position determination in cobalt coordinated pentasaccharides using ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 9, 1125–1134 (1998)
Gaucher, S.P., Leary, J.A.: Determination anomericity of the glycosidic bond in Zn (II)-diethylenetriamine–disaccharide complex using MSn in a quadrupole ion trap. J. Am. Soc. Mass Spectrom. 10, 269–272 (1999)
Weiskopf, A.S., Vouros, P., Harvey, D.J.: Characterization of oligosaccharide composition and structure by quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 11, 1493–1504 (1997)
Weiskopf, A.S., Vouros, P., Harvey, D.J.: Electrospray ionization-ion trap mass spectrometry for structural analysis of complex N-linked glycoprotein oligosaccharides. Anal. Chem. 70, 4441–4447 (1998)
Viseux, N., de Hoffmann, E., Domon, B.: Structural assignment of permethylated oligosaccharide subunits using sequential tandem mass spectrometry. Anal. Chem. 70, 4951–4959 (1998)
Viseux, N., Costello, C.E., Domon, B.: Post-source decay mass spectrometry: Optimized calibration procedure and structural characterization of permethylated oligosaccharides. J. Mass Spectrom. 34, 364–378 (1999)
Prien, J.M., Ashline, D.J., Lapadula, A.J., Zhang, H., Reinhold, V.N.: The high mannose glycans from Bovine Ribonuclease B isomer characterization by ion trap MS. J. Am. Soc. Mass Spectrom. 20, 539–556 (2009)
Ahn, Y.H., Yoo, J.S.: Malononitrile as a new derivatizing reagent for high-sensitivity analysis of oligosaccharides by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 12, 2011–2015 (1998)
Li, D.T., Her, G.R.: Structural analysis of chromophore-labeled disaccharides and oligosaccharides by electrospray ionization mass spectrometry and high-performance liquid chromatography/electrospray ionization mass spectrometry. J. Mass Spectrom. 33, 644–652 (1998)
Charlwood, J., Langridge, J., Tolson, D., Birrell, H., Camilleri, P.: Profiling of 2-aminoacridone derivatized glycans by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 13, 107–112 (1999)
Saba, J.A., Shen, X., Jamieson, J.C., Perreault, H.: Effect of 1-phenyl-3- methyl-5-pyrazolone labeling on the fragmentation behavior of asialo and sialated n-linked glycans under electrospray ionization conditions. Rapid Commun. Mass Spectrom. 13, 704–711 (1999)
Shen, X., Perreault, H.: Electrospray ionization mass spectrometry of 1-phenyl-3-methyl-5-pyrazolone derivatives of neutral and n-acetylated oligosaccharides. J. Mass Spectrom. 34, 502–510 (1999)
Harvey, D.J.: Electrospray mass spectrometry and fragmentation of n-linked carbohydrates derivatized at the reducing terminus. J. Am. Soc. Mass Spectrom. 11, 900–915 (2000)
Li, D.T., Sheen, J.F., Her, G.R.: Structural analysis of chromophore-labeled disaccharides by capillary electrophoresis tandem mass spectrometry using ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 11, 292–300 (2000)
Cheng, H.L., Her, G.R.: Determination of linkages of linear and branched oligosaccharides using closed-ring chromophore labeling and negative ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 1322–1330 (2002)
Cheng, H.L., Pai, P.J., Her, G.R.: Linkage and branch determination of N-linked oligosaccharides using sequential degradation/closed-ring chromophore labeling/negative ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 248–259 (2007)
Harvey, D.J.: Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J. Chromatogr. B 879, 1196–1225 (2011)
Mechref, Y., Novotny, M.V., Krishnan, C.: Structural characterization of oligosaccharides using MALDI-TOF/TOF tandem mass spectrometry. Anal. Chem. 75, 4895–4903 (2003)
Spina, E., Sturiale, L., Romeo, D., Impallomeni, G., Garozzo, D., Waidelich, D., Glueckmann, M.: New fragmentation mechanisms in matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry of carbohydrates. Rapid Commun. Mass Spectrom. 18, 392–398 (2004)
Stephens, E., Maslen, S.L., Green, L.G., Williams, D.H.: Fragmentation characteristics of neutral n-linked glycans using a MALDI-TOF/TOF tandem mass spectrometer. Anal. Chem. 76, 2343–2354 (2004)
Zaia, J.: Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 23, 161–227 (2004)
Wuhrer, M., Deelder, A.M.: Negative-mode MALDI-TOF/TOF-MS of oligosaccharides labeled with 2-aminobenzamide. Anal. Chem. 77, 6954–6959 (2005)
Morelle, W., Slomianny, M.C., Diemer, H., Schaeffer, C., Dorsselaer, A.V., Michalski, J.C.: Structural characterization of 2-aminobenzamide-derivatized oligosaccharides using a matrix-assisted laser desorption/ionization two-stage time-of-flight tandem mass spectrometer. Rapid Commun. Mass Spectrom. 19, 2075–2084 (2005)
Lewandrowski, U., Resemann, A., Sickmann, A.: Laser-induced dissociation/high-energy collision-induced dissociation fragmentation using MALDI-TOF/TOF-MS instrumentation for the analysis of neutral and acidic oligosaccharides. Anal. Chem. 77, 3274–3283 (2005)
Mechref, Y., Kang, P., Novotny, M.V.: Differentiating structural isomers of sialylated glycans by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20, 1381–1389 (2006)
Domon, B., Mueller, D.R., Richter, W.J.: Tandem mass spectrometric analysis of fixed-charge derivatized oligosaccharides. Org. Mass Spectrom. 29, 713–719 (1994)
Packer, N.H., Lawson, M.A., Jardine, D.R., Redmond, J.W.: A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj. J. 15, 737–747 (1998)
Lai, C.C., Her, G.R.: Analysis of N-glycosylation of phospholipase A2 from venom of individual bees by microbore high performance liquid chromatography/electrospray mass spectrometry using an ion trap mass spectrometer. J. Chromatogr. B 766, 243–250 (2002)
Fu, D., Chen, L., O'Neill, R.A.: A detailed structural characterization of ribonuclease B oligosaccharides by 1H NMR spectroscopy and mass spectrometry. Carbohydr. Res. 261, 173–186 (1994)
Domon, B., Costello, C.E.: A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5, 397–409 (1988)
Acknowledgments
The authors acknowledge support for this work by the National Research Council of the Republic of China.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 719 kb)
Rights and permissions
About this article
Cite this article
Chen, ST., Her, GR. Linkage and Branch Analysis of High-Mannose Oligosaccharides Using Closed-Ring Labeling of 8-Aminopyrene-1,3,6-Trisulfonate and P-Aminobenzoic Ethyl Ester and Negative Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 23, 1408–1418 (2012). https://doi.org/10.1007/s13361-012-0420-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-012-0420-0