Abstract
Introduction
Sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors inhibit the reabsorption of glucose from the kidneys and increase urinary glucose excretion (UGE), thereby lowering the blood glucose concentration in people suffering from type 1 and type 2 diabetes mellitus (T2DM). In a previous study, we reported a pharmacokinetics/pharmacodynamics model to estimate individual change in UGE (ΔUGE), which is a direct pharmacological effect of SGLT2 inhibitors. In this study, we report our enhancement of the previous model to predict the long-term effects of ipragliflozin on clinical outcomes in patients with T2DM.
Methods
The time course of fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) in patients with T2DM following ipragliflozin treatment that had been observed in earlier clinical trials was modeled using empirical models combined with the maximum drug effect (Emax) model and disease progression model. As a predictive factor of drug effect, estimated ΔUGE was introduced into the Emax model, instead of ipragliflozin exposure. The developed models were used to simulate the time course of FPG and HbA1c following once-daily treatment with placebo or ipragliflozin at doses of 12.5, 25, 50 and 100 mg, and the changes at 52 weeks at the approved dose of 50 mg were summarized by renal function category.
Results
The developed models that included UGE as a dependent variable of response were found to well describe observed time courses in FPG and HbA1c. Baseline blood glucose level and renal function had significant effects on the glucose-lowering effect of ipragliflozin, and these models enabled quantification of these impacts on clinical outcomes. Simulated median changes in HbA1c in T2DM patients with mild and moderate renal impairment were 25 and 63% lower, respectively, than those in T2DM patients with normal renal function. These results are consistent with the observed clinical data from a previous renal impairment study.
Conclusions
Empirical models established based on the effect of UGE well predicted the renal function-dependent long-term glucose-lowering effects of ipragliflozin in patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In a previous study, we developed a model to estimate the change in urinary glucose excretion (UGE) as a pharmacological effect of sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors. |
This earlier model explained weaker glucose-lowering effects of ipragliflozin in patients with renal impairment caused by decreased glucose filtration. |
In this study, we enhanced the model to predict the long-term time course in fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) after treatment with SGLT2 inhibitor. |
What was learned from the study? |
The newly constructed FPG and HbA1c models well predicted the renal function-dependent long-term glucose-lowering effects of ipragliflozin in patients with type 2 diabetes mellitus. |
The results using these models suggest that baseline blood control and renal function have a significant impact on the glucose-lowering effects of ipragliflozin, and the models enabled quantification of this impact. |
Introduction
Sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors are a novel class of drugs that inhibit the reabsorption of glucose in the kidneys and therefore increase urinary glucose excretion [1]. Ipragliflozin (Suglat®) is a selective SGLT2 inhibitor [2] co-developed by Astellas Pharma Inc. (Tokyo, Japan) and Kotobuki Pharmaceutical Co., Ltd. (Nagano, Japan) for the treatment of type 1 (T1DM) and type 2 diabetes mellitus (T2DM) and has been approved in Japan, Korea and Russia. The recommended dosage is 50 mg once daily before or after breakfast. Combination therapy with several antihyperglycemic agents has been approved. When efficacy is insufficient, dose increase up to 100 mg, once daily, is allowed in Japan and Russia.
In our previous study, we established a pharmacokinetics/pharmacodynamics (PK/PD) model of ipragliflozin to describe the relationship between the daily exposure in the plasma concentration of ipragliflozin [area under the concentration–time curve in 24 h (AUC24h)] and the increase in urinary glucose excretion (UGE) in 24 h (UGE24h) [3]. The maximum effect of SGLT2 inhibitors on UGE never exceeds the filtered glucose level, which in turn depends on the plasma glucose level and renal function. The model enabled individual predictions of the AUC24h of ipragliflozin and UGE24h at steady-state with two significant predictors, namely, the individual fasting plasma glucose level (FPG) and glomerular filtration rate (GFR).
The aim of the present study was to expand the previous PK/PD model to enable prediction of the long-term glucose-lowering effects of ipragliflozin in patients with T2DM and to identify and quantify the impact of factors influencing the glucose-lowering effects of ipragliflozin.
Methods
Study Design
An outline of the clinical studies previously conducted on ipragliflozin that were used in this study is presented in Table 1. A phase I study in healthy subjects (Study A) and two clinical pharmacology studies in T2DM patients (Studies B and C) were used to develop the PK/PD model established previously [3].
A total of 5893 FPG and 5371 HbA1c data points were obtained from 834 patients with T2DM in four late-phase clinical studies (Studies D–G). Observations of FPG and HbA1c from three studies (Studies D–F) that examined once-daily oral administration in T2DM patients were used to characterize models of the glucose-lowering effects of ipragliflozin. Data from a long-term study in renal impairment patients (Study G) were excluded from the model building but were used for simulation as external validation of the developed model. In Study G only, a concomitant dose of one other oral hypoglycemic agent was allowed, and the baseline plasma glucose level was significantly lower than that in the other studies. After building the model, the long-term antihyperglycemic effects were simulated using demographic data from all 887 patients with T2DM in the studies.
All clinical trial studies were conducted in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. An independent ethics committee or institutional review board approved the clinical protocol at each participating center. All participants provided written informed consent prior to inclusion.
Model Building
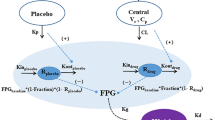
To describe the time course of FPG and HbA1c in patients with T2DM after treatment with ipragliflozin (EFFt), a combined empirical model with disease progression (St) and the drug effect of ipragliflozin (DEt) was developed separately for FPG and HbA1c, with reference to Frey’s model [11]. The equation of the combined model is as follows:
where EFFt is the estimated FPG or HbA1c at time t, BASE is the FPG or HbA1c value at baseline and εprop is a proportional residual error of FPG or HbA1c at each time point. To avoid overestimating disease progression, outliers were identified using a quantile–quantile plot of the change in HbA1c from baseline, and FPG and HbA1c records with a change in HbA1c from baseline that exceeded ± 1.0% for placebo or + 1.0% for active treatment at each visit were excluded from the analysis prior to modeling.
The natural time course of disease progression (St) was modeled using an exponential function and/or a linear increase slope. The glucose-lowering effects of ipragliflozin as change from baseline (DEt) were described using a maximum drug effect (Emax) model with an effect compartment according to the following equation:
The effect of baseline FPG (FPGB) or HbA1c (HBA1CB) was incorporated into the Emax of ipragliflozin using the power function because exploratory plots of the change from baseline versus baseline value indicated the significant impact of baseline values on Emax in both the FPG and HbA1c models. The effect compartment was employed to describe a delay in reaching the maximum effect. The individual ΔUGE24h predictions from the previous model [3] were introduced in the effect compartment, instead of drug exposure, as a main predictor of response because the UGE effect seemed to be directly linked to the glucose-lowering effect in plasma. The time course of the effect compartment was described using the rate constant of equilibration (Keq) and maximum time of assessment period (T = 52 weeks).
The inter-individual variability (IIV) was parametrized for EC50 (amount of ΔUGE24h where 50% of its maximal effect is observed) and model parameters to describe disease progression curves. Details for the model building steps are shown in the Electronic Supplementary Material (ESM) files.
Model Evaluation
Models were assessed using goodness-of-fit (GOF) plots. Predictive performance of the final model was evaluated using a prediction-corrected visual prediction check (VPC) with 1000 generated datasets. Robustness of the final model was assessed using 1000 runs with the non-parametric bootstrap method.
Simulation
The time course of changes in FPG and HbA1c following once-daily treatment with placebo or ipragliflozin at 12.5, 25, 50, and 100 mg were simulated for 887 Japanese patients with T2DM enrolled in the six clinical studies (Studies B–G). In addition, the relationship among the simulated AUC24h of the plasma ipragliflozin concentration, ΔUGE24h and treatment effects represented by changes in FPG and HbA1c from baseline (ΔFPG and ΔHbA1c) at 52 weeks were summarized according to dose regime and subgroup stratified by renal function. Simulation results were summarized as median and prediction interval (PI).
Software
All statistical data processing and summarization were performed using SAS® version 9.1 software (SAS Institute, Inc., Cary, NC, USA) and R version 2.13.1 or subsequent versions (The R Foundation for Statistical Computing, Vienna, Austria). All NONMEM analysis was performed using the first-order conditional estimation method with interaction (FOCE-I) on NONMEM® version 7.3.0.
Results
Demographics and Laboratory Variables
A summary of patients’ demographics and clinical laboratory variables are presented in Table 2. The estimated GFR (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) study equation modified for the Japanese general population [12], and body surface area was calculated using the Du Bois equation [13].
Exploratory Assessment
A total of 3021 FPG and 2674 HbA1c data points from 589 patients with T2DM in studies D–F were included in the analysis. Data from patients who were administered an increased ipragliflozin dose during the study were excluded from the analysis. Outliers were identified, and 131 records (approximately 5% of the total records) for observations of FPG or HbA1c were excluded from the analysis in advance.
Models for FPG
The parameter estimates in the final model are presented in ESM Table S1. The time course of changes in FPG was described using a combination of disease progression and drug effect based on data from the placebo arms and active treatment arms.
Disease progression during inactive treatment was described as:
An exponential function was applied to describe the observed disease progression in inactive treatment because greater progression occurred in the first 4–8 weeks. A large IIV was observed for the disease progression curve.
The drug effect in terms of change from baseline in FPG was described as:
The population mean Emax was 45.3 mg/dL at the reference value of FPGB (162 mg/dL), and the baseline FPG level had the greatest effect on Emax. The model suggests that patients with 10% higher FPG levels at baseline have 25% larger Emax values. The population mean EC50 and the IIV were 39.4 g per 24 h and 277% (coefficient of variation [CV] %), respectively. The large variation suggested significant IIV in drug sensitivity. The estimated equilibration constant (Keq) used to describe the delay in response was 0.283 per week, suggesting that the time to reach the maximum response was approximately 12 weeks at fivefold an equilibration half-life equal to ln(2)/Keq. The estimated residual error of FPG at each time point was 28.2% (CV %).
Models for HbA1c
The parameter estimates in the final model are presented in ESM Table S2. Disease progression was described as:
As for FPG, a large IIV was observed for the disease progression curve of HbA1c in the first 4–8 weeks. Furthermore, the gradual HbA1c increase after long-term treatment was observed by visual inspection; therefore, a linear long-term disease progression slope (0.0123 per week) was added in the HbA1c model only.
Drug effect, expressed as the change from baseline in HbA1c, was described as:
In the HbA1c model, an exponential function of ∆UGE24h,t was ineffective for describing the delay in achieving the maximum effect. Instead, the linear function was used to describe the amount of the effect compartment. The population mean Emax at the reference value of HbA1c (7.9%) was 1.56%, and the baseline HbA1c level had the most influence on Emax. The model suggests that patients with 10% higher HbA1c at baseline have 34% larger Emax values. The population mean EC50 and the IIV was 24.1 g/24h and 90.0% (CV %), respectively. The estimated residual error of HbA1c at each time point was 15.3% (CV %).
Model Evaluation
The GOF plots suggested that the final FPG and HbA1c models were adequate. The conditional weighted residuals (CWRES) showed no trend with dose, study or visit (ESM Figs. S1, S2). VPC plots demonstrated that the final models were able to reproduce the observed data regardless of treatment (ESM Figs. S3, S4). The summary statistics of the bootstrap estimates were consistent with the parameter estimates of the final models, suggesting the robustness of the estimates (ESM Tables S1, S2).
Simulation
Simulated median values and the 95% PI (2.5th–97.5th percentile) of exposure (AUC24h), PD effect (ΔUGE24h) and the clinical endpoints (ΔFPG and ΔHbA1c) for each dose regimen are summarized in Fig. 1. Following once-daily doses of placebo or ipragliflozin at 50 and 100 mg, simulated values of ΔFPG were + 5.7 (− 24.2 to 36.0), − 24.0 (− 101.3 to 9.1) and − 24.9 (− 103.0 to 8.8) mg/dL, respectively, and simulated ΔHbA1c values were + 0.64% (− 0.45 to 1.70%), − 0.65% (− 2.38 to 0.67%) and − 0.67% (− 2.44 to 0.63%), respectively.
Simulated exposure–response at steady-state. The red line is the median of prediction. The pink zone is the 95% prediction interval (2.5th–97.5th percentile). AUC Area under the concentration–time curve, ΔFPG, ΔHbA1c change in fasting plasma glucose and hemoglobin A1c from baseline at 52 weeks, ΔUGE change in urinary glucose excretion in 24 h from baseline
The simulated time course of changes in FPG and HbA1c according to renal function suggested that patients with T2DM with moderate renal impairment show a smaller mean decrease in both FPG and HbA1c than their counterparts with normal or mild renal impairment (Fig. 2). The influence of renal function on AUC24h, ΔUGE24h, ΔFPG and ΔHbA1c is summarized in Table 3. The simulated median ΔHbA1c at 52 weeks in T2DM patients with mild and moderate renal impairment was 25 and 63% lower, respectively, than that in patients with normal renal function.
Simulated time course of changes in fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) according to renal function (eGFR estimated glomerular filtration rate [units: mL/min/1.73 m2]). Circles represent observations. The black dashed line is the Lowess line of the individual observations. The gray zone represents the 95% confidence interval of the Lowess line of the individual observations. The red line is the median of prediction. The pink zone is the 95% prediction interval (2.5th–97.5th percentile)
Discussion
The established model was found to well describe the time course of changes in FPG and HbA1c observed in clinical trial studies involving patients on ipragliflozin. Although the empirical models have some limitations in exploring the long-term progression of T2DM, we demonstrated that the constructed model provides useful predictions of the blood glucose-lowering effects of ipragliflozin on the clinical endpoints.
The United Kingdom Prospective Diabetes Study 33 (UKPDS 33) followed the natural disease progression of T2DM patients for more than 10 years [14] and revealed an apparently linear increase in FPG and HbA1c with time. In the developed model described here, the linear disease progression slope for HbA1c increase was 0.0123% per week (0.64% per year). This estimation was twofold higher than that reported by Mahesh [15], which was based on data from two long-term trials, A Diabetes Outcome Progression Trial (ADOPT) [16] and the UKPDS trials [14]. Considering the differences in patient background, study period and modeling approach, the magnitude of this difference in the rate of disease progression is not particularly large. The established model implicitly indicates that the gradual HbA1c increase observed during long-term treatment with ipragliflozin was not caused by a time-dependent decrease in or tolerance of drug effect.
A phase II study (Study D) showed dose-dependent reductions in both FPG and HbA1c, with the observed mean change in HbA1c from baseline at 12 weeks after treatment being + 0.50% in the placebo group and − 0.11, − 0.47, − 0.79 and − 0.81% in the ipragliflozin 12.5, 25, 50 and 100 mg groups, respectively [7]. In a phase III study (Study E), the observed mean change in HbA1c from baseline at 16 weeks after treatment was + 0.54 and − 0.76% in the placebo and ipragliflozin 50 mg groups, respectively [8]. In all phase III studies, the effect on FPG and HbA1c consistently reached the maximum level at 12–16 weeks and 20–24 weeks after starting ipragliflozin treatment, respectively [8,9,10]. The estimated rate constant of equilibration (Keq) for ΔFPG also suggested that the time to reach the maximum level was approximately 12 weeks, which was comparable to the reported value for gliclazide [11].
The strong correlations of ΔFPG or ΔHbA1c with the corresponding baseline values, and the model estimations for Emax change suggested that the baseline blood glucose level was most influential parameter on the blood glucose-lowering effect of ipragliflozin. Renal function also had a significant impact on the drug effect, while other factors (age, gender, among others) had only a minimal effect on ΔFPG and ΔHbA1c. In a long-term renal impairment study (Study G), weaker glucose-lowering effects were observed in those patients with moderate renal impairment compared to patients with normal renal function (mean change from baseline: − 13 mg/dL in FPG and − 0.44% in HbA1c at 52 weeks in the 50 mg once-daily group) [17]. Similar trends were also reported in a long-term study of other SGLT2 inhibitors in T2DM patients with renal impairment [18,19,20]. Therefore, in Emax models for clinical outcome, we have applied estimated ΔUGE instead of drug exposure, which was calculated by considering the effect of renal function [3].
We summarized the reported long-term clinical outcomes in empagliflozin, canagliflozin and dapagliflozin [18,19,20] using data from two ipragliflozin studies [9, 10]. Mean changes in HbA1c at 52 weeks after treatment were plotted against a multiplied parameter of FPG and GFR at baseline, using each individual patient’s data in the ipragliflozin studies (Fig. 3). As a result, a linear decreasing trend in HbA1c was observed when plotted against the amount of glucose filtration as a multiplied parameter of FPG and GFR. The mean trend found in ipragliflozin studies was fully consistent with the evidence confirmed in clinical trials with other SGLT2 inhibitors [18,19,20]. According to the mechanism of action of SGLT2 inhibitors, the maximum effect of SGLT2 inhibitors on the UGE never exceeds the amount of filtered glucose [21], and a weaker effect by a SGLT2 inhibitor is expected due to a lower maximum amount of filtered glucose in this particular population. The summary of clinical trial outcomes supports the rationale of using simulated ΔUGE24h values as a predictor in our models. Furthermore, the summary plot based on our modeling knowledge suggests that all SGLT2 inhibitors will show similar clinical outcomes at the same blood glucose level and renal function background. The assessment of renal function is recommended before and after treatment of SGLT2 inhibitors in the respective package inserts, and the use of a SGLT2 inhibitor in patients with moderate renal function is not recommended due to a lack of efficacy. Dose increase in patients with renal impairment should be approached with caution because no further effect is expected in terms of mechanism of action. Conversely, a large glucose-lowering effect is expected in patients with normal renal function and high blood glucose level. Our model also suggests that the IIV of time to maximum treatment effect is large due to the difference in disease progression and drug sensitivity. Given the variability of response time, any dose increase of ipragliflozin should be considered after at least 3 months of treatment.
Relationship between amount of filtered glucose (GFR*FPG) at baseline and long-term glucose-lowering effects of the sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor. Circles show the individual outcomes after long-term treatment of patients with type 2 diabetes mellitus (T2DM) receiving once-daily doses of ipragliflozin at 50 mg (Studies F and G). The red line shows the Lowess line of the individual outcomes following ipragliflozin treatment. Results of each clinical trial are plotted. The size of the symbols represents the proportional weight of the sample size of each subgroup according to renal impairment stage: normal (eGFR ≥ 90 mL/min/1.73 m2), stage 2 (eGFR 60 to < 90 mL/min/1.73 m2), stage 3 (eGFR 30 to < 60 mL/min/1.73 m2) and stage 4 (eGFR < 30 mL/min/1.73 m2). Clinical trials: diamond indicates treatment with empagliflozin 25 mg in T2DM patients with stage 2–4 chronic kidney disease (ClinicalTrials.gov Identifier: NCT01164501) [18]; triangle indicates treatment with canagliflozin 300 mg in T2DM patients with stage 3 chronic kidney disease (NCT01064414) [19]; filled circle indicates treatment with dapagliflozin 10 mg in T2DM patients in a phase 2/3 study (NCT00663260) [20]; square indicates treatment with ipragliflozin 50 mg in T2DM patients with normal renal function (Study F; NCT01054092) [9] and with renal impairment (Study G; NCT01316094) [10]
A limitation of the present analysis is that the model estimates for the disease progression, drug effect or impact of demographic factors on the drug effect fluctuate in the population with different treatment backgrounds (e.g. use of concomitant medication, drug naive or not, etc.). Extrapolation of further long-term outcomes from the model is less robust.
Conclusions
The constructed models predict the long-term changes in FPG and HbA1c in individuals with T2DM based on the pharmacological effects of SGLT2 inhibitors related to increases in UGE. These models enable quantification of the impact of baseline glucose control and renal function on the glucose-lowering effects of ipragliflozin.
References
Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract. 2008;62(8):1279–84.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(4):423–36.
Saito M, Kaibara A, Kadokura T, Toyoshima J, Yoshida S, Kazuta K, et al. Pharmacokinetic and pharmacodynamic modeling for renal function dependent urinary glucose excretion effect of ipragliflozin, a selective sodium-glucose cotransporter 2 inhibitor, both in healthy subjects and patients with type 2 diabetes mellitus. Br J Clin Pharmacol. 2019;85(8):1808–19.
Kadokura T, Saito M, Utsuno A, Kazuta K, Yoshida S, Kawasaki S, et al. Ipragliflozin (ASP1941), a selective sodium-dependent glucose cotransporter 2 inhibitor, safely stimulates urinary glucose excretion without inducing hypoglycemia in healthy Japanese subjects. Diabetol Int. 2011;2:172–82.
Kadokura T, Akiyama N, Kashiwagi A, Utsuno A, Kazuta K, Yoshida S, et al. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106(1):50–6.
Ferrannini E, Veltkamp SA, Smulders RA, Kadokura T. Renal glucose handling: impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013;36(5):1260–5.
Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(4):382–91.
Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycaemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6:8–18.
Kashiwagi A, Kawano H, Kazuta K, Utsuno A, Yoshida S. Long-term safety, tolerability and efficacy of ipragliflozin in Japanese patients with type 2 diabetes mellitus—IGNITE study. Jpn Pharmacol Ther. 2015;43(1):85–100. http://www.lifescience.co.jp/yk/yk15/jan/ab8.html.
Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17(2):152–60.
Frey N, Laveille C, Paraire M, Francillard M, Holford NH, Jochemsen R. Population PKPD modelling of the long-term hypoglycaemic effect of gliclazide given as a once-a-day modified release (MR) formulation. Br J Clin Pharmacol. 2003;55(2):147–57.
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11(1):41–50.
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5(5):303–11.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Samtani MN. Simple pharmacometric tools for oral anti-diabetic drug development: competitive landscape for oral non-insulin therapies in type 2 diabetes. Biopharm Drug Dispos. 2010;31(2–3):162–77.
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43.
Clinical study report synopsis. 2019. https://astellasclinicalstudyresults.com/hcp/docs/1941-CL-0072/Redacted%20Synopsis/1941-cl-0072-clgr-disc01-en-final-02.pdf. Accessed 14 Feb 2020.
Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–84.
Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. DIA3004 Study Group. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16(10):1016–27.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–71.
Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–70.
Acknowledgements
Ipragliflozin (Suglat®) was co-developed by Astellas Pharma Inc. and Kotobuki Pharmaceutical Co., Ltd. All studies and analyses were funded by Astellas Pharma Inc. The authors thank all of the investigators involved in each trial.
Funding
This study and the journal’s Rapid Service were funded by Astellas Pharma Inc.
Medical Writing Assistance
Medical writing and editorial support was funded by Astellas Pharma Inc. and was provided by Heidi Tran, PhD, of DMC Corp. www.dmed.co.jp and Goto M. of EMC KK.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) authorship guidelines for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were involved with drafting and revising this article. MS, AK and TK planned the analysis, and MS conducted the analysis. JT contributed to data verification and the creation of tables and figures. SY was the lead statistician responsible for data handling in each study. KK was the study leader for ipragliflozin studies and contributed to the planning and conduct of the clinical studies. EU was the project manager for ipragliflozin studies and contributed to mapping the development strategy.
Disclosures
Atsunori Kaibara is a former full-time employee of Astellas Pharma Inc., Tokyo, Japan, and currently a full-time employee of Eli Lilly Japan K.K., Tokyo, Japan. Masako Saito, Takeshi Kadokura, Junko Toyoshima, Satoshi Yoshida, Kenichi Kazuta and Eiji Ueyamaare are employees of Astellas Pharma Inc., Tokyo, Japan.
Compliance with Ethics Guidelines
All clinical trial studies were conducted in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. An independent ethics committee or institutional review board approved the clinical protocol at each participating center. All participants provided written informed consent prior to inclusion.
Data Availability
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx NONMEM modeling codes are included in supplementary material.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11778873.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/
About this article
Cite this article
Saito, M., Kaibara, A., Kadokura, T. et al. Model-based Prediction of the Long-term Glucose-Lowering Effects of Ipragliflozin, a Selective Sodium–Glucose Cotransporter 2 (SGLT2) Inhibitor, in Patients with Type 2 Diabetes Mellitus. Diabetes Ther 11, 951–964 (2020). https://doi.org/10.1007/s13300-020-00785-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00785-2