Abstract
Background
This phase 1, randomized, placebo-controlled, dose-escalation study evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of ipragliflozin (ASP1941) in healthy Japanese subjects.
Methods
Subjects received a single oral dose (1–300 mg) of ipragliflozin or multiple once-daily oral doses (20–100 mg) for 7 days. The effect of food on pharmacokinetics and pharmacodynamics was explored by administering a single dose of 100 mg in the fasting and fed states. Adverse events were recorded throughout the study.
Results
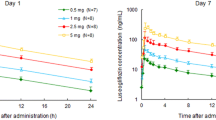
Ipragliflozin was well tolerated. Adverse events were mild, none was hypoglycemia related, and no urinary or genital infections were reported. Ipragliflozin was rapidly absorbed, reaching maximum plasma concentration within 3 h. Maximum plasma concentration and area under the plasma concentration-time curve increased dose-proportionally. Plasma half-life was reached 10.0–13.3 h after the first dose of ipragliflozin ≥3 mg, and no dose-dependent relationship was observed. Food had no significant impact on the pharmacokinetics and pharmacodynamics of ipragliflozin. Plasma glucose levels were not significantly affected by ipragliflozin administration. Urinary glucose excretion increased dose-dependently. A maximum of approximately 70 and 50 g of glucose excreted over 24 h was reached after single 300 mg dosing and after multiple 50 or 100 mg dosing, respectively.
Conclusions
Ipragliflozin was well tolerated and stimulated dose-dependent increases in urinary glucose excretion in healthy Japanese subjects.




Similar content being viewed by others
References
van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3–8.
International Diabetes Federation. IDF Diabetes Atlas. 4th ed. 2009. http://www.diabetesatlas.org. Accessed 9 Mar 2011.
Nakano T, Ito H. Epidemiology of diabetes mellitus in old age in Japan. Diabetes Res Clin Pract. 2007;77(Suppl 1):S76–81.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
Crofford OB. Diabetes control and complications. Annu Rev Med. 1995;46:267–79.
Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42.
Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30.
Hollander PA, Kushner P. Type 2 diabetes comorbidities and treatment challenges: rationale for DPP-4 inhibitors. Postgrad Med. 2010;122:71–80.
Moss SE, Klein R, Klein BE, Meuer SM. The association of glycemia and cause-specific mortality in a diabetic population. Arch Intern Med. 1994;154:2473–9.
Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–68.
Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract. 2008;62:1279–84.
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for d-glucose. J Clin Invest. 1994;93:397–404.
Neumiller JJ, White J R Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010;70:377–85.
Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95:34–42.
Kurosaki E, Tahara A, Yokono M, et al. In vitro and in vivo pharmacological properties of ASP1941, a novel, potent and selective SGLT2 inhibitor. Orlando: American Diabetes Association; 25–29 Jun 2010 (abstract 0570-P).
Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Diabetol Int. 2010;1:2–20.
Bailey CF, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–33.
List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–7.
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24.
Veltkamp SA, Kadokura T, Krauwinkel WJJ, Smulders RA. ASP1941, a novel and selective SGLT2 inhibitor, stimulates urinary glucose excretion in healthy subjects. Orlando: American Diabetes Association; 25–29 Jun 2010 (abstract 0565-P).
Oba K, Igari Y, Matsumura N, et al. Effects of control of blood glucose on urinary excretion of N-acetyl-beta-D-glucosaminidase in elderly type 2 diabetes mellitus. J Nippon Med Sch. 2000;67:143–5.
Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–6.
Hussey EK, Clark RV, Amin DM, et al. Single-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy volunteers and patients with type 2 diabetes mellitus. J Clin Pharmacol. 2010;50:623–35.
Kapur A, O’Connor-Semmes RL, Hussey EK, et al. First human dose escalation study with remogliflozin etabonate (RE) in healthy subjects and in subjects with type 2 diabetes mellitus. New Orleans: American Diabetes Association; 5–9 Jun 2009 (abstract 509-P).
Kolodny EH, Kline R, Altszuler N. Effect of phlorizin on hepatic glucose output. Am J Physiol. 1962;202:149–54.
Acknowledgments
Ipragliflozin is in clinical development by Astellas Pharma Inc. and Kotobuki Pharmaceutical Co., Ltd. The authors thank Sandra Mendes, PhD, from Excerpta Medica, who provided writing/editorial assistance for this work, which was supported by Astellas. This study was funded by Astellas Pharma Inc.
Conflict of interest
Takeshi Kadokura, Masako Saito, Atsushi Utsuno, Kenichi Kazuta, Satoshi Yoshida, Shigenori Kawasaki, and Itsuro Nagase are employees of Astellas Pharma Inc., Tokyo, Japan. Shigeru Kageyama is a medical advisor for ipragliflozin, developed by Astellas Pharma Inc., Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinicaltrials.gov: NCT01121198 (1941-CL-0101).
About this article
Cite this article
Kadokura, T., Saito, M., Utsuno, A. et al. Ipragliflozin (ASP1941), a selective sodium-dependent glucose cotransporter 2 inhibitor, safely stimulates urinary glucose excretion without inducing hypoglycemia in healthy Japanese subjects. Diabetol Int 2, 172–182 (2011). https://doi.org/10.1007/s13340-011-0037-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-011-0037-8