Abstract
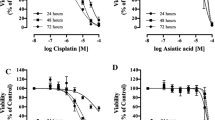
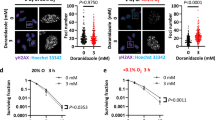
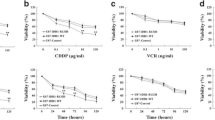
Resistance to drugs, which is aggravated by hypoxia, is a well-known feature of tumors. The combination of drug exposure and hypoxia can give rise to several survival strategies in the exposed cells. Glioblastoma multiforme (GBM) is among the most hypoxic of solid tumors, and we have used glial cells to identify a drug combination that would be synergistically effective in these cells under both normoxia and hypoxia. Cisplatin (CP) and 2-deoxy-d-glucose (2-DG), which have been used for second-line therapy and for preclinical research, are relatively ineffective as single agents. During in vitro experiments with A172 and LN229 cells, there was increased resistance to both drugs under hypoxia. However, the combination of CP and 2-DG showed a synergistic effect in reducing cell viability under both normoxia and hypoxia, with a combination index of less than 1. Increased autophagy is a distinct feature of the response to 2-DG. However, autophagic markers were reduced, and apoptotic markers were upregulated by the combination, indicating a switch over from autophagic to apoptotic pathways with reduction in endoplasmic reticulum (ER) stress. The combination also resulted in a decrease of pAKT levels. The effect of CP in the combination was replicated by the prototype AKT inhibitor LY294002, further supporting the role of AKT inhibition in the synergism. Combination of 2-DG with CP, or possibly an AKT inhibitor, can prove to be an effective rational combination for reducing chemoresistance under both normoxic and hypoxic conditions in gliomas.








Similar content being viewed by others
Abbreviations
- GBM:
-
Glioblastoma multiforme
- UPR:
-
Unfolded protein response
- 2-DG:
-
2-Deoxy-d-glucose
- CP:
-
Cisplatin
- ER:
-
Endoplasmic reticulum
- ATP:
-
Adenosine triphosphate
- AMPK:
-
AMP-activated protein kinase
- TMZ:
-
Temozolomide
- ATCC:
-
American Type Culture Collection
- CI:
-
Combination index
- 3-MA:
-
3-Methyladenine
- CQ:
-
Chloroquine
References
Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30(7):679–92.
Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10:8177–84.
Das G, Shravage BV, Baehrecke EH. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4(6).
Codogno P. Autophagy in cell survival and death. J Soc Biol. 2005;199(3):233–41.
Rzymski T, Milani M, Singleton DC, Harris AL. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle. 2009;8(23):3838–47.
Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–906.
Jang M, Kim SS, Lee J. Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med. 2013;45:e45.
Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-d-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355(2):176–83.
Dwarakanath B, Jain V. Targeting glucose metabolism with 2-deoxy-d-glucose for improving cancer therapy. Future Oncol. 2009;5(5):581–5.
Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, et al. 2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol. 2011;67:899–910.
Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ. Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry. 2001;40:5542–7.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014:364–78.
Datta K, Shah P, Srivastava T, Mathur SG, Chattopadhyay P, Sinha S. Sensitizing glioma cells to cisplatin by abrogating the p53 response with antisense oligonucleotides. Cancer Gene Ther. 2004;11(8):525–31.
Ahmad M, Hahn IF, Chatterjee S. GRP78 up-regulation leads to hypersensitization to cisplatin in A549 lung cancer cells. Anticancer Res. 2014;34(7):3493–500.
Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, Simões-Wüst AP, Yousefi S, Simon HU, Stahel R, Zangemeister-Wittke U. Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int J Cancer. 2005;117(5):755–63.
Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, Zhou W. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008;7(4):809–17.
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–67.
Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, Zhou L, Liao J, Keihack H, Yan L, Rubin E, Yang JM. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther. 2012;11(1):154–64.
Jin R, Nakada M, Teng L, Furuta T, Sabit H, Hayashi Y, Demuth T, Hirao A, Sato H, Zhao G, Hamada J. Combination therapy using Notch and Akt inhibitors is effective for suppressing invasion but not proliferation in glioma cells. Neurosci Lett. 2013;534:316–21.
Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65(16):7429–35.
Wu Y, Sarkissyan M, Mcghee E, Lee S, Vadgama JV. Combined inhibition of glycolysis and AMPK induces synergistic breast cancer cell killing. Breast Cancer Res Treat. 2015;151(3):529–39.
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–6.
Zhao L, Au JL, Wientjes MG. Comparison of methods for evaluating drug-drug interaction. Front Biosci (Elite Ed). 2010;2:241–9.
Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-d-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67(7):3364–70.
Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, Aghi MK. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72(7):1773–83.
Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29(10):2570–81.
Ramirez-Peinado S, Leon-Annicchiarico CL, Galindo-Moreno J, Iurlaro R, Caro-Maldonado A, Prehn JH, Ryan KM, Munoz-Peinado C. Glucose-starved cells do not engage in pro-survival autophagy. J Biol Chem. 2013;288(42):30387–98.
Moruno-Manchón JF, Pérez-Jiménez E, Knecht E. Glucose induces autophagy under starvation conditions by a p38 MAPK-dependent pathway. Biochem J. 2013;449(2):497–506.
Schlie K, Spowart JE, Hughson LR, Townsend KN, Lum JJ. When cells suffocate: autophagy in cancer and immune cells under low oxygen. Int J Cell Biol. 2011;2011:470597.
Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-d-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One. 2011;6(2):e17234.
DeSalvo J, Kuznetsov JN, Du J, Leclerc GM, Leclerc GJ, Lampidis TJ, Barredo JC. Inhibition of Akt potentiates 2-DG-induced apoptosis via downregulation of UPR in acute lymphoblastic leukemia. Mol Cancer Res. 2012;10(7):969–78.
Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793(4):664–73.
Jiang K, Li Y, Zhu Q, Xu J, Wang Y, Deng W, Liu Q, Zhang G, Meng S. Pharmacological modulation of autophagy enhances Newcastle disease virus-mediated oncolysis in drug-resistant lung cancer cells. BMC Cancer. 2014;14:551.
Shchors K, Massaras A, Hanahan D. Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit. Cancer Cell. 2015;28(4):456–71.
Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, Banerjee AK, Das S, Jena A, Ravichandran R, Sahi UP, Kumar R, Kapoor N, Kalia VK, Dwarakanath BS, Jain V. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35(1):103–11.
Acknowledgments
The study has been supported by core institutional grant of the National Brain Research Centre (NBRC), India to Prof. Subrata Sinha. Fellowships provided by the Indian Council of Medical Research (ICMR) to Akansha Jalota and Mukesh Kumar are acknowledged. Prof. B. C. Das is thankful to the Department of Science and Technology (DST) for the award of J C Bose fellowship and Dr. Ajay Kumar Yadav to the Department of Biotechnology (DBT) for Ramalingaswamy fellowship. The technical support provided by Ms. Jyoti, Mr. Pappu Prasad, and Mr. Mukesh is deeply acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
ESM 1
(DOC 31 kb)S
Rights and permissions
About this article
Cite this article
Jalota, A., Kumar, M., Das, B.C. et al. Synergistic increase in efficacy of a combination of 2-deoxy-d-glucose and cisplatin in normoxia and hypoxia: switch from autophagy to apoptosis. Tumor Biol. 37, 12347–12358 (2016). https://doi.org/10.1007/s13277-016-5089-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5089-8