Abstract
Objectives
To compare the effectiveness, side effects, and patient satisfaction of buccal versus vaginal misoprostol administration in first and second trimester induced abortions.
Methods
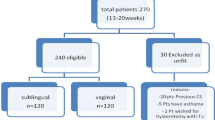
In first trimester, women received oral mifepristone followed by misoprostol either by buccal or vaginal route. In second trimester, women received oral mifepristone followed by repeated doses of misoprostol either by buccal or vaginal route. A comparative analysis using SPSS was done.
Results
In first trimester, success rate of medical abortion was 96 % in buccal group and 88 % in vaginal group. Nausea was the most common adverse effect which was similar in both groups. In second trimester, success rate was 96 % in buccal group and 80 % in vaginal group. A statistically higher incidence of nausea was noticed in buccal group. Patient satisfaction level was almost similar in both the groups in both trimesters.
Conclusions
Buccal and vaginal routes of misoprostol administration have similar efficacy and patient satisfaction level for first and second trimester induced abortions. Hence, buccal route may serve as an alternative to vaginal misoprostol.
Similar content being viewed by others
References
Medical Termination of Pregnancy act. MTP act (1971) and MTP rule (1972): Govt. of India.
Lohr PA, Reeves MF, Hayes JL, et al. Oral mifepristone and buccal misoprostol administered simultaneously for abortion: a pilot study. Contraception. 2007;76:215–20.
Middleton T, Schaff E, Fielding SL, et al. Randomized trial of mifepristone and buccal or vaginal misoprostol for abortion through 56 days of last menstrual period. Contraception. 2005;72:328–32.
Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71:22–5.
Medical management of first-trimester abortion. Practice Bulletin No. 143. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:676–92.
Fjerstad M, Sinin I, Lichtenberg ES, et al. Effectiveness of medical abortion with mifepristone and buccal misoprostol through 59 gestational days. Contraception. 2009;80:282–6.
Wildschut H, Both MI, Medema S, et al. Medical methods for mid-trimester termination of pregnancy. Cochrane Database Syst Rev. 2011;2:CD005216.
WHO. Safe abortion: technical and policy guidance for health systems. Geneva: WHO; 2003.
RCOG. The care of women requesting induced abortion. London: Royal College of Obstetricians and Gynaecologists; 2004.
Ellis SC, Kapp N, Vragpvoc O, et al. Randomized trial of buccal versus vaginal misoprostol for induction of second trimester abortion. Contraception. 2010;81:441–5.
Daponte A, Nzewenga G, Dimopoulos KD, et al. The use of vaginal misoprostol for second-trimester pregnancy termination in women with previous single cesarean section. Contraception. 2006;74:324–7.
Berghella V, Airoldi J, O’Neill AM, et al. Misoprostol for second trimester pregnancy termination in women with prior caesarean: a systematic review. BJOG. 2009;116(9):1151–7.
Compliance with ethical standards
The study was conducted among the patients selected from out patient department of Government Medical College and Hospital, Sector 32, Chandigarh and requesting for medical abortions. A written and informed consent was taken from all. The interventions involved in the present study are routinely practiced in Obstetrics and Gynecology and are safe. The patients were given the right to opt out of the study at any time they want. The defined guidelines of Central Ethics Committee for Biomedical Research on Human subjects by ICMR and guidelines as per Helsinki Declaration were strictly adherent in the present project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, G., Takkar, N. & Sehgal, A. Buccal Versus Vaginal Misoprostol Administration for the Induction of First and Second Trimester Abortions. J Obstet Gynecol India 65, 111–116 (2015). https://doi.org/10.1007/s13224-014-0605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-014-0605-5