Abstract
This study demonstrates the preparation of heterogeneous ZnO: Co nanostructures via hydrothermal–electrochemical deposition at 130 °C and −1.1 V (vs Ag/AgCl (satd)) in dimethyl sulfoxide (DMSO)–H2O mixture. Under the stated conditions, ZnO: Co nanostructures grow preferentially along (002) direction. Strength of directional growth progressively increases with the increasing concentration of Co(II) in the deposition bath. Films are composed of hexagonal Wurtzite ZnO, metallic cobalt, and mixed cobalt oxide on the surface and cobalt(II) oxide in deeper levels. Increasing the Co(II) concentration in the deposition bath results in different morphological features as well as phase separation. Platelets, sponge-like structures, cobalt-rich spheres, microislands of cobalt-rich spheres which are interconnected by ZnO network can be synthesized by adjusting [Co(II)]: [Zn(II)] ratio. Growth mechanisms giving rise to these particular structures, surface morphology, crystal structure, phase purity, chemical binding characteristics, and optical properties of the deposits are discussed in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc oxide (ZnO) is one of the most promising materials in the materials research and device fabrication technologies, with a wide range of potential applications from sensors to optoelectronic devices such as lasers, light emitting diodes, cantilevers, and solar cells (Schmidt-Mende and Macmanus-Driscoll 2007). This attraction stems from its wide bandgap (3.37 eV), large exciton binding energy (60 meV), optical transparency, thermal and chemical stability, and fast electron transfer kinetics (Look 2001). Moreover, electrical and optical properties of ZnO particles can be modified to broaden possible applications through impurity doping. To date, considerable attention has been paid to metal doping. Studies on Mn2+,Cu2+, Cd2+, Ni2+,Mg2+, Fe2+, and particularly on Co2+-doped ZnO have been reported (Ohtomo et al. 1998; Matei et al. 2010; Yin et al. 2004; Liu et al. 2008; Lawes et al. 2005; Jug and Tikhomirov 2009). Among these materials, Co-doped ZnO crystals receive growing attention (Matei et al. 2010; Liu et al. 2008; Chang et al. 2012; Bohle and Spina 2010; Yao and Zeng 2009). Although engineering the bandgap of ZnO crystals with Co doping has been shown to alter the electronic properties compared to the parent materials, the results are controversial. For example, the defect related visible emissions of ZnO has been reported to be both enhanced (Chang et al. 2012) and quenched (Bohle and Spina 2010) with increasing cobalt amount in the structure. Consequently, understanding the influence of cobalt dopant on the optical properties of ZnO remains as an open question.
Some of the methods that were reported to obtain Co: ZnO/ZnO: Co particles include spray pyrolysis (Zhou et al. 2007), chemical vapor deposition (Tuan et al. 2004), magnetron sputtering (Song et al. 2007), solution based approaches (Yao and Zeng 2009; Sesha et al. 2012; Panigrahy et al. 2010), pulsed laser deposition (Ivill et al. 2008) and electrodeposition (Ou et al. 2010). Electrochemical synthesis is a simple, cheap and efficient technique which allows production of large area-thin films on various substrates at relatively lower temperatures. Furthermore, control over growth rate, film thickness and particle morphology can be easily maintained by manipulation of deposition potential, current, temperature, and precursor concentration. Film properties can also be tuned controlling type and concentration of additives and substrate type. Examples include formation of dendritic CoO/ZnO alloys (Ou et al. 2010) and hexagonal ringlike superstructures of Co-doped ZnO nanorods (Li et al. 2008). Electrodeposition from a bath containing Zn(NO3)2, Co(NO3)2, NH4F, and citric acid at 90 °C results in formation of dendrite-like arrangement of periodic CoO/ZnO alloy hexagonal nanoplatelets (Ou et al. 2010). A combination of electrochemistry with hydrothermal synthesis technique at 90 °C, on the other hand, was shown to produce hierarchical Co-doped ZnO hexagonal superstructures that are composed of nanorods (Li et al. 2008). Although many of the deposition parameters vary tremendously, the reported temperatures for electrochemical synthesis of ZnO mostly range between 60 and 90 °C, below 100 °C, which is the limit for aqueous reactions. However, Park et al. have demonstrated that a combination of hydrothermal and electrochemical methods yields ZnO nanorods with better optical properties deposited at higher temperatures such as 120–180 °C (Park et al. 2009; Park et al. 2012). Recently, we have extended the studies on the effects of hydrothermal–electrochemical deposition (HED) at higher temperatures (130 °C) on physical and chemical properties of metal oxide particles. HED primarily affects the growth kinetics; hence, crystallinity and morphology of deposited particles (Yilmaz and Unal 2015). Furthermore, by the synergistic effect of the growth-modifier molecules and HED, metal oxide particles with various shapes including spherical-, rhombohedral-, and platelet-like particles can be obtained (Yilmaz and Unal 2013; Yilmaz and Unal 2016; Yilmaz and Unal 2014). Effect of additives is significantly influenced by high-temperature electrodeposition with each cation and anion behaving differently. It has been presented that by adjusting [MnCl2] in the deposition bath, diameters of ZnO rods can be tuned by HED (Yilmaz and Unal 2013). In the following study, we have shown that besides precursor concentration, identities of the additive metal cation and counter ion in the deposition solution are important to produce ZnO particles with various morphologies. Hierarchical ZnO architectures can be obtained at a single step via HED by modifying [Cd(CH3COO)2/Zn2+] in the deposition bath, which are not formed by electrodeposition at 70–90 °C (Yilmaz and Unal 2014). In this study, the effects of the presence of Co2+ ions in the deposition solution under conditions of 130 °C and 2 bars on the morphology and physical structure of ZnO films have been investigated. We report the growth of heterogeneous ZnO: Co nanostructures by seedless hydrothermal–electrochemical deposition at 130 °C from dimethyl sulfoxide (DMSO)–water mixture, for the first time. Herein, the effect of cobalt ion amount on the morphology and physical properties of the resulting films is discussed in detail.
Materials and methods
Materials
All the chemicals used were of analytical grade. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O) was obtained from Merck, and zinc nitrate hexahydrate (Zn(NO3)2·6H2O) was purchased from Sigma-Aldrich. Dimethyl sulfoxide (DMSO, C2H6OS) was supplied by Alfa Aesar. Indium tin oxide (ITO) [Ω < 5.0 × 10−4 O cm (R s <100 O/sq)] was purchased from Teknoma Ltd. Izmir, Turkey. Double distilled, high-purity water was used from Milli-Q water (millipore) system.
Synthesis and characterization
ZnO: Co layers were grown on ITO (Indium tin oxide) coated glass electrode (1 × 1 cm2 area) in 50 ml of 1:1 (v:v) DMSO: H2O mixture. A conventional 3-electrode cell system in a hydrothermal glass reactor (Büchiglasuster, modified picoclave) was used for electrochemical deposition. The volume of the reactor was 100 ml. The reference electrode was Ag/AgCl saturated with KCl (Corr Instruments, S/N P11092) whereas Pt wire served as the counter electrode. The distance between the electrodes is approximately 2 cm. The substrate was cleaned ultrasonically by ethanol, acetone, and distilled water prior to depositions. The thin films were cathodically electrodeposited from baths containing Zn(NO3)2 and Co(NO3)2. The Zn(II) concentration was fixed to 50 mM and [Co(II)]\[Zn(II)] ratio was varied between 0.2 and 1. Details of the synthesis parameters are provided in Table 1. The pH of the solutions is varied between 5 and 7. Electrodepositions were carried out at constant potential of −1.1 V vs Ag/AgCl at 130 °C for 30 min (unless stated otherwise) on a potentiostat/galvanostat (Biologic-Science Instruments, VSP model) which resulted in approximately tens of micrometers thick films (Fig. S1 in the supporting information).
The crystal structure and crystallinity of the samples were analyzed by X-Ray diffraction (XRD) using Bruker/D8 advance with Cu-Kα radiation. The surface morphologies of the films were examined using ZEISS ultra plus field emission scanning electron microscope (FE-SEM). Zn–Co surface distribution was profiled using Bruker energy dispersive spectrometer (EDX) attached to ZEISS ultra-plus field emission scanning electron microscope (FE-SEM). The film composition was studied by X-ray photoelectron spectroscopy (Thermo K-Alpha, XPS). C1s (285 Ev) peak served as a reference to calibrate the binding energies. Depth-profile analysis was performed by etching the surface with Ar+. Photoluminescence (PL) spectra of the films were recorded using Horiba Jobin–Yvon–Fluoromax 3 spectrofluorometer between 375 and 700 nm at 355 nm excitation wavelength. Raman scattering experiments were carried out using a Renishaw Raman microscope system at room temperature. 532-nm line was used for excitation.
Results and discussion
Morphology and structure
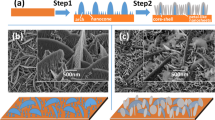
Electron microscopy was used to investigate the effect of cobalt doping on the morphology of the films. SEM images of the as-prepared heterogeneous ZnO: Co films with the increasing Co(II) amount in the electrodeposition bath are displayed in Fig. 1. A deposition bath containing only 50 mM Zn(NO3)2 in 1:1 DMSO: H2O mixture gives rise to the formation of thin platelets with about 1–2-µm-sized edges as shown in our previous study (Yilmaz and Unal 2013). Similar morphology was observed for the film grown from the bath containing Zn(NO3)2 and Co(NO3)2 with a [Co(II)]: [Zn(II)] ratio of 0.2. However, addition of Co(II) into the deposition solution resulted in an increase in the ZnO platelet size. Platelets with diameters of about 2–4 µm, which were grown perpendicular to the surface, are observed in the low-magnification image (Fig. 1a). High-magnification image shows the presence of a network of porous, sponge-like structures among these plates (Fig. 1b). EDX analysis of the film revealed that this porous network grew in between the ZnO platelets were rich in cobalt (Fig. S2 in the supporting information). As concentration of Co(II) was increased further, spheres with diameters of 6–8 µm were observed on an interconnected-network of platelets (Fig. 1c). EDX results indicated that cobalt-rich spheres that were composed of aggregated plate-like particles were formed on a matrix of ZnO: Co platelets (Fig. S3 in the supporting information). When [Co(II)]/[Zn(II)] ratio was 0.6, a clear phase separation was observed, where cobalt-rich islands develop on the ZnO: Co matrix (Fig. 1d). Further Co(II) addition into the deposition bath resulted in an increased concentration of cobalt-rich spheres (covered with zinc-rich platelets)(Fig. 1e) and the porous network on the phase-separated matrix (Fig. 1f) as seen in SEM figures and EDX analysis (Fig. S4 in the supporting information).
The structure and phase of the ZnO: Co films were determined by X-ray diffraction (Fig. 2). When [Co(II)]/[Zn(II)] ratio was smallest, three major peaks that can be indexed to hexagonal wurtzite ZnO (JCDPDS No.0361451) were present at 2 = 31.7°, 34.4°, and 36.2° corresponding to (100), (200), and (101) reflections, respectively. Relative higher intensity of (100) and (101) reflections according to (200) peak intensity is consistent with the observed plate morphology of the films. Similar patterns were reported for disk and wall shaped ZnO structures (Pradhan and Leung 2008). Furthermore, three more reflections arising at 2 = 41.67°, 44.5°, and 47.46° were detected which showed the existence of metallic cobalt formation in the form of hexagonal closed-pack (hcp) or face-centered cubic (fcc) crystal structure or a mixture of both (Pan et al. 2010; Capitaneo et al. 2006). As the concentration of Co(II) in the electrodeposition bath was increased further, a preferential growth along the perpendicular direction to the substrate surface was observed as pointed out by the highly intense (002) peak. The strong (002) peak indicates formation of highly crystalline, well-aligned ZnO particles. This further suggests that the presence of cobalt(II) in the mother liquor does not alter the crystallinity of the ZnO films. Furthermore, cobalt(II) addition into the deposition solution did not induce shift in the position of (002) reflection regardless of Co(II) concentration. This behavior was reported earlier by Tortosa et al. for Zn1−x Co x O films electrodeposited from DMSO solution (Tortosa et al. 2008). They suggested that, similar to Cd-doped ZnO films, the potential changes in the unit cell volume and cell parameters due to metal doping only affect the direction perpendicular to the c axis but not the c axis parameter. It might be also due to low doping levels since it was already established for Cd-doped ZnO films that doping levels lower than 9% does not induce any change in the lattice parameters (Tortosa et al. 2007). In addition, similar radii of the two cations (0.58 Å for Co2+ and 0.6 Å for Zn2+) (Wang et al. 2007) might also account for the lack of peak shift. Crystal sizes calculated by Debye–Scherrer equation using (002) peak does not display significant change with cobalt(II) amount in solution (3–4 nm), but they are all larger than the calculated crystal size of undoped ZnO (2.5 nm) which is also supported by SEM images.
XRD patterns of films prepared from baths with different feed ratios: a [Co(II)]: [Zn(II)] = 0.2, b [Co(II)]: [Zn(II)] = 0.4, c [Co(II)]: [Zn(II)] = 0.6, d [Co(II)]: [Zn(II)] = 0.8, e [Co(II)]: [Zn(II)] = 1. The (asterisk) symbol represents reflections associated with substrate ITO. The scale bars represent 2000 counts unless stated otherwise.)
XPS
X-ray photoelectron spectroscopy was used to study both the nature of chemical bonding states of the metals and the composition of the films. Figure 3 displays surface Co 2p XPS spectra of the ZnO: Co films as well as the spectra from deeper levels after etching. For the film with the lowest [Co(II)/[Zn(II)] feed ratio, after surface etching, the Co 2p 3/2 and Co 2p 1/2 peaks were located at 780.7 and 796.5 eV, respectively, and separated by 15.8 eV. Two strong satellite bands at approximately 6 eV higher binding energies accompanied the main peaks. These features imply the presence of oxidized cobalt in the form of Co2+ in a tetrahedral crystal field surrounded by oxygens (Yin et al. 2004; Kim 1975). Strong satellite peaks are attributed to the charge-transfer structure characteristics of 3d transition metal (TM) monoxides. On the other hand, the surface of the film was characterized by two main peaks separated by 14.9 eV, which appeared at 781.7 and 796.6 eV. The main peak at 781.7 eV was sharper and the satellites were wider and of lower intensity than those of the peaks observed after surface etching. These aspects of the lines suggest the presence of cobalt oxide as Co3O4 where Co2+ and Co3+ located at tetrahedral and octahedral sites, respectively, or mixed oxide with a valence state of +3 for cobalt in an octahedral geometry on the surface of the film (Yin et al. 2004; Jiménez et al. 1998). Even though XRD analysis indicated the presence of metallic cobalt for the film with the lowest [Co(II)]/[Zn(II)] ratio, no peaks associated with cobalt metal was detected on the XPS spectra. Metallic cobalt might be located in levels deeper than the detectable depth of the XPS measurement. The ligand field splitting energy of octahedral geometry is lower than the tetrahedral one. Hence, the color of appearance is shifted to lower energies. The films appear in color range of dark-brown to black which is consistent with what is reported for Co3O4 before (Xia et al. 2010). When the cobalt(II) amount in the electrodeposition solution was increased further to deliver [Co(II)]/[Zn(II)] feed ratio of 0.4, main Co 2p 1/2 peak on the surface was shifted to 795.8 eV and Co 2p 3/2 peak was shifted to 779.9 eV. Main peaks appeared with two satellites at binding energies of 786.4 and 789.9 eV, all again consistent with Co3O4 on the surface (Hagelin-weaver et al. 2004). After surface etching of 540 s, a similar transformation from Co3O4 to CoO was observed. The main Co 2p 1/2 and Co 2p 3/2 peaks shifted to higher binding energies of 0.4–0.6 eV. The satellite peak at 789.9 eV disappeared, and the satellite intensities for both Co 2p 1/2 and Co 2p 3/2 increased to be higher than 50% of the main peaks as could be expected to be observed in CoO spectrum (Yin et al. 2004; Hagelin-weaver et al. 2004). The film prepared with a [Co(II)]/[Zn(II)] feed ratio of 0.8 displayed a very similar XPS spectra as the film prepared by the lowest cobalt(II) amount. When the [Co(II)]/[Zn(II)] feed ratio ranges between 0.6 and 1, the cobalt region of the XPS spectra showed a distinct feature. The surfaces of the films demonstrated Co3O4-like character as the other films. However, after etching the surface for 540 s, two additional peaks located at 778.4 and 793.5 eV appeared which correspond to the Co 2p 1/2 and Co 2p 3/2 peaks of metallic cobalt, respectively (Yang et al. 2010; Mclntyre and Cook 1975). Following half-cell reactions can account for reduction and oxidation of Co2+ ions (Santamaria et al. 2013):
Co 2p XPS spectra of the ZnO: Co films prepared with a feed ratios of a [Co(II)]: [Zn(II)] = 0.2, b [Co(II)]: [Zn(II)] = 0.4, c [Co(II)]: [Zn(II)] = 0.6, and d [Co(II)]: [Zn(II)] = 0.8, f [Co(II)]: [Zn(II)] = 1. Line 1 shows XPS spectrum from surface, and line 2 displays the XPS spectrum after 540 s of etching
The pH range in which ZnO is thermodynamically stable was calculated to be above 6.10 at 298 K and above 5.28 at 333 K by Otani et al. (2006). E eq values for reaction 1b are 0.622, 0.150, 0.587, and 0.150 V (vs Ag/AgCl), respectively, under conditions: [Co2+] = 0.05 M at pH = 5; [Co2+] = 0.05 M at pH = 7; [Co2+] = 0.02 M at pH = 5; and [Co2+] = 0.02 M at pH = 7. Hence, formation of metallic cobalt and mixed cobalt oxide is thermodynamically possible under applied potential of −1.1 V (Ag/AgCl).
Figure 4 shows the XPS spectra of the O1s region (bottom) and Zn 2p region (top) from the surface of the film prepared with the lowest cobalt(II) amount and after surface etching. The O1s spectra are asymmetric, and the deconvoluted peaks exhibit intensity changes over etching time. On the surface of the film, the peaks appear at 531.4 and 529.8 eV. The peak localized at lower energy is usually attributed to O2− ions in the wurtzite crystal structure of hexagonal ZnO lattice (Tam et al. 2006). The peak at higher energy, on the other hand, usually is reported to result from the presence of loosely bound oxygen or OH− species on the surface, or O2− ions in the oxygen deficient regions (Hsieh et al. 2008). The intensity of this defect-related peak decreases significantly after etching. This behavior suggests that oxygen vacancies/hydroxides are present mostly on the surface. In addition, the intensity of the peak that can be indexed to Zn–O bond is amplified in the inner layers. The spectrum of the Zn 2p region displays two peaks centered at 1021.4 and 1045.3 eV which could be assigned to Zn 2p 3/2 and 2p 1/2 of Zn2+ in ZnO, respectively (Fig. 4, top). These lines are not shifted after etching process, which implies good chemical stability of Zn throughout the film. Similar trend is observed for all of the ZnO: Co films for O1s and Zn 2p regions of the XPS spectra (data not shown).
Raman
Raman spectroscopy has been used to understand consequences of impurity doping on host lattice characteristics, and modification of lattice vibrational modes, as well as to investigate secondary phase formation that might not be detectable by conventional characterization methods like XRD due to detectability limit of the measurement (Chang et al. 2012; Zhou et al. 2007; Thakur et al. 2007). Substitution of the dopant atoms would introduce additional vibrational modes. Wurtzite crystal structure of ZnO belongs to space group 4C6v for which Raman active zone-center optical modes with symmetries A 1 + 2B 1 + E 1 + 2E 2 are predicted by group theory. Out of these modes, A 1 and E 1 phonons are polar modes, which split into transverse-optical (TO) and longitudinal-optical (LO) phonon modes at different energies, and all are both Raman= and IR-active modes. The nonpolar E 2(low) and E 2(high) are attributed to the vibration of zinc sublattice and oxygen motion, respectively (Wang et al. 2007; Thakur et al. 2007). The B 1 mode, on the other hand, is silent. Raman spectra obtained at room temperature for pure ZnO films and the as-prepared ZnO: Co films deposited from baths with different [Co(II)]: [Zn(II)] feed ratios in the range of 150 and 1000 cm−1 are displayed in Fig. 5. The broad, low-intensity peak at 331 cm−1 and the peaks observed at 378 and 436 cm−1 for pure ZnO can be indexed to the second-order scattering, A 1(TO), and E 2(high), respectively; where E 2(high) mode dominates and indicates highly crystalline wurtzite ZnO (Chang et al. 2012; Sesha et al. 2012; Wang et al. 2007). The frequency of 331 cm−1 mode matches with the difference of E 2(high)−E 2(low) modes. Cusco et al. analyzed this second-order mode by temperature-dependent Raman studies and assigned the symmetry of this mode to be composed of predominantly A 1, with a smaller E 2 component and an even smaller E 1 component (Cuscó et al. 2007). The comparatively weak Raman band at 484 cm−1 is assigned as surface-optical phonon modes of highly c axis oriented ZnO nanorods in the literature which is observed when the size of the crystals is much smaller than the wavelength of the incident light (Gupta et al. 2006; Milekhin et al. 2012). The absence of E 1(TO) mode at 410 cm−1 further supports the preferential growth through c axis (Gupta et al. 2006) in line with XRD data.
When Co(II) was introduced into the electrodeposition solution, Raman spectrum of the film deposited displayed additional bands together with alterations in ZnO frequencies. The high intensity E 2(high) mode of ZnO was blueshifted in frequency to 427 cm−1 as well as two-phonon-difference mode (from 333 to 321 cm−1). The E 2(high) mode of ZnO films has been found to be predominantly due to oxygen atom motions through isotopic mass dependence measurements of the E 2 phonons (Serrano et al. 2003). The asymmetric line shape and frequency of this mode are shown to be sensitive to oxygen mass fluctuations. Hence, the blueshift in the frequency of the E 2(high) mode might stem from the oxygen vacancies which would create oxygen mass loss depending on the abundance (Thakur et al. 2007). Furthermore, the blueshift and the broadening of this mode also suggest structural defect formation and lattice distortions due to cobalt doping (Wang et al. 2007). In addition, compared with pure ZnO Raman spectra, rather broad and low-intensity bands appeared at 197, 464, 522, and 662 cm−1. The peaks at 197 and 522 cm−1 were blueshifted to 185 and 510 cm−1, respectively, and the peak at 662 cm−1 narrowed down to be centered at 669 cm−1 as the amount of the introduced Co(II) was increased. The peaks at 185, 464, 510, and 669 cm−1 can be assigned to F 2g(1), E g, F 2g(2), and A 1g phonon modes of Co3O4, respectively (Wang et al. 2007). Co3O4 belongs to Fd3m space group for which Γ = A 1g + E g + F 1g + 3F 2g + 2A 2u + 2E u + 4F 1u + 2F 2u modes are predicted by group theory. Out of these vibrations, A 1g, E g, and 3F 2g are Raman active; 4F 1u is Infrared active; and F 1g, A 2u, E u, and F 2u are silent. The peak for A 1g mode strengthened, and was redshifted in frequency as the Co(II) amount increased. This change might be due to the increased octahedral/Co3+ (CoO6) subunit concentration, since A 1g mode is stimulated by octahedral oxygen motion (Wang et al. 2007; Jiang and Li 2007). The F 2g(1) mode, on the other hand, is ascribed to be excited by tetrahedral sites (CoO4) (Jiang and Li 2007). The appearance of Co3O4-like modes with [Co(II)]: [Zn(II)] feed ratio of as low as 0.2 implies that the second-spinel phase begins to form as soon as cobalt(II) is introduced into the system, as also confirmed by the XPS analysis. When the [Co(II)]: [Zn(II)] feed ratio was raised to 0.6 and higher, Co3O4 overwhelmed the Wurtzite structure, and the E 2(high) and A 1(TO) modes were diminished significantly.
Crystallization
Deposition of cobalt-rich oxide spheres on ZnO: Co matrix might be explained by the electrochemical reactions as follows, in accordance with XRD data. Electroreduction of the nitrate ions in the growth solution results in formation of the hydroxide ions, which increases the pH (Eq. 3). The hydroxide ions in turn react with Zn2+ ions to cause precipitation of zinc hydroxide (Eq. 4; hydroxylation) at the cathode which is subsequently dehydrated to generate ZnO (Eq. 5; dehydration).
Wurtzite ZnO structure is composed of O2−-terminated and Zn2+-terminated layers stacked on top of each other along the c axis (Ozgur et al. 2005). This arrangement of the atoms allowed ZnO possess high-energy polar planes such as ±(0001) which are the primary growth surfaces and six symmetric, nonpolar {1010} planes parallel to the c axis. Hence, incoming precursors tend to add on the polar surfaces of a newly formed ZnO seed leading to a faster growth along ±[0001] direction, which actually is shown to be five times faster than the growth on the {1010} and {0110} planes (Pauporte et al. 2002). This anisotropic growth behavior gives rise to particles growing with relatively high aspect ratios. However, it is possible to modify the morphology of the particles by preventing the growth on particular surfaces using selectivity of the surfaces limited to surfactants and capping agents. The morphology of the polar inorganic crystals can also be controlled by taking advantage of their sensitivity to reaction solvents (Xu and Wang 2011). Solvent polarity and saturated vapor pressure play important roles on the growth behaviors through crystal–solvent interfacial interactions (Zhang 2002). Preferred growth direction is hindered in the case of strongly polar solvents/molecules since they interact with the polar surfaces and hinder the direct growth on nonpolar planes. Being a polar, basic, aprotic solvent, DMSO is also responsible for the anisotropic growth of ZnO structures (Lu et al. 2009; Zhai et al. 2012). A mass of DMSO is most likely absorbed on to the polar basal plane of ZnO inhibiting the absorption of additional growth units on (0001) plane. As a result, growth proceeds along six symmetric nonpolar planes, and ZnO platelets were obtained in the absence of Co(II) ions in DMSO–H2O mixture (Yilmaz and Unal 2013). Besides morphological control, DMSO undergoes hydrolysis in aqueous solutions and also contributes toward increasing the OH− ion concentration according to the following equation (Rodríguez-Gattorno et al. 2003):
Higher OH− concentration, in turn, accelerates the hydroxylation reaction (Eq. 4) further; hence, the nucleation rate increases. Enhanced nucleation rate would result in the formation of amplified number of primary nuclei adjacent to each other, which might account for the intermittent particle growth. In addition, it is known that DMSO undergoes decomposition to yield sulfur at elevated temperatures (Elbaum et al. 2001). Since the reactions were carried out at 130 °C and at the corresponding vapor pressure, DMSO decomposition was expected. Production of sulfur was confirmed by XPS analysis which implies the presence of sulfur as sulfide incorporated in the films (Fig. S5 in the supporting information).
Addition of Co(II) into the deposition solution significantly affected the crystal growth and hence morphology of the obtained products. To better understand formation of spheres and the observed phase separation as explained in the previous sections (Fig. 1), the particle growth was analyzed through controlled experiments over different deposition times at selected [Co(II)]/[Zn(II)] ratios. Analyses of XRD and XPS data of the films prepared with varying deposition times revealed some growth behaviors. First of all, metallic cobalt formation was detected to occur early in the reaction. In the XRD diagram of the film with [Co(II)]: [Zn(II)] = 0.8 (Fig. 6), metallic cobalt reflections at 2 = 41.6°, 44.5°, and 47.5° were present even after first 5 min of the deposition. Early metallic cobalt formation was also verified by analysis of XPS spectra (Fig. S6 in the supporting information). Peaks that correspond to binding energy of cobalt metal appeared also for the films with [Co(II)]: [Zn(II)] = 0.6 and [Co(II)]: [Zn(II)] = 0.4 with shorter reaction times after 480 s of etching (Fig. S6 in the supporting information). The presence of cobalt metal only in deeper levels of the film explains why it could not be detected in the XPS spectra of the thicker films (30 min.) as discussed above.
In addition, hydroxide-to-oxide transformation consistent with the deposition mechanism was observed during film growth as hydroxide peak (531.4 eV) intensity relative to the oxide peak (530.1 eV) in XPS spectra (Fig. 7) of the film with [Co(II)]: [Zn(II)] = 0.8 was decreased with the increasing deposition time. Finally, highly preferential ZnO growth along c axis was observed. After first 5 min of deposition, three major hexagonal wurtzite ZnO reflections [(100), (200), and (101)] were present in the XRD diagram of the film with [Co(II)]: [Zn(II)] = 0.8 (Fig. 6). Intensity of (002) reflection was progressively enhanced at the expense of (100) and (101) peaks over time and became the major diffraction line after 30 min, indicating the preferential growth vertical to the substrate surface.
In light of the above analysis, for the films prepared with [Co(II)]: [Zn(II)] ratios of 0.4 and higher, it can be concluded that cobalt-rich phase (mainly oxide according to the XPS data (Fig. S6 in the supporting information), and Zn(OH)2-rich matrix form in the first 10 min of the reaction. As the reaction proceeds and more OH− is produced, Zn(OH)2 continues to grow both on the initial Zn(OH)2 nuclei and in between the cobalt-rich islands so that Zn(OH)2 has grown beneath cobalt-rich oxide spheres which is then converted to ZnO later in time. When [Co(II)]: [Zn(II)] ratio is raised to 0.6 and higher, the number of the initial cobalt-rich spheres is amplified (Figs. 1d, 8). This increase suggests that the cobalt-rich phase grows faster than the zinc-rich phase. EDX data clearly show that spheres are composed mainly of cobalt-rich oxides (Fig. 8). O1s XPS data for this film confirm that the topmost layer is composed of zinc hydroxides as expected. Removing upper layers by Ar+ etching reveals the ZnO layer beneath which is in accordance with the crystallization mechanism shown in Eqs. 4, 5. In the end, a hierarchical assembly is constructed where ZnO rods are the core building blocks that carry cobalt-rich oxide plates, which form the roots of the cobalt-rich-cobalt oxide spheres after 30 min of deposition (Fig. S7 in the supporting information). The spheres arrange together to form microislands which are interconnected by ZnO network (Fig. 1d).
Hence, examination of the initial and later stages of the reaction suggests growth of the particles occurs through Ostwald Ripening (OR) mechanism, which has been widely proposed for the growth of spherical particles and ZnO structures. In addition, the fact that numerous platelets form the spheres, reveals oriented attachment mechanism (OA) (Xu and Wang 2011; Li et al. 2013; Yu et al. 2007; Zhang et al. 2011; Zhang et al. 2008). The initial Zn(OH)2 and cobalt-rich nuclei (poorly crystallized particles characterized by low-intensity diffraction peaks) are unstable due to large surface area exposed, and to minimize the interfacial energy, they tend to aggregate so that spheres form out of platelets. Then, irregularities due to oriented aggregation is removed to produce round and smooth particles through OR mechanism (Li et al. 2013). At this stage, Zn(OH)2 and cobalt-rich grains grow further locally at the expense of smaller nuclei [in the perpendicular direction to the film surface depicted by highly intense (002) reflection] and tend to fuse by decreasing the gap between as seen in SEM images after 30 min of deposition, especially when Co(II) amount is higher.
Total charge passed through the systems during depositions was calculated from current–time curves and are summarized in Table 1. The total charge passed and, hence the current increase with the increasing Co(II) amount in the deposition solutions. It is also noticeable that this increase in the total charge is correlated with the increase in the nitrate amount. Hence, it can be concluded that this parameter monitors the reduction of nitrate ions (Eq. 2), and the increase corresponds to an increase of the rate of this reaction with the increasing Co(II) concentration.
In a previous study regarding synthesis of mesoporous ZnO microspheres with a hydrothermal process, it was documented that higher temperatures (80 °C) induce more ZnO microsphere formation by aggregation of plate-like structures (Zhang et al. 2011). Hydrothermal treatment improves OR process by facilitating growth of larger particles via dissolution of smaller particles and suggested to be a flourishing environment to obtain special structures. Hence, electrodeposition in a hydrothermal environment at a higher temperature (130 °C) than conventional systems might have prompted in suggesting OR as the growth mechanism.
It was observed for all samples that Co-rich spheres were grown on a zinc hydroxide layer in the initial stages of deposition. The amount of Co, however, played a crucial role in the determination of the final morphology. As [Co(II)] amount was increased, phase separation was observed: the degree of which was positively correlated with Co concentration (Fig. 1). When Co concentration was increased further, both the sizes and the amounts of the Co-rich spheres increased, and some were fused together. Moreover, zinc oxide platelets continued to grow under and around Co-rich phase so that Co-rich spheres were covered with zinc oxide platelets.
Photoluminescence
Finally, the effect of cobalt doping on the emission properties of ZnO films was analyzed by photoluminescence (PL) measurements. Room-temperature PL spectra of pure ZnO film and the ZnO: Co films with [Co(II)]: [Zn(II)] = 0.2 and 0.4 are shown in Fig. 9. A strong transition at around 370–385 nm was observed in all of the films resulting from the direct recombination of the hole and the excited electron known as near-band-edge (NBE) emission. The luminescence peak at 385 nm is the ZnO emission. NBE emission of the pure ZnO film was shifted to higher energies for ZnO: Co films due to Burstein–Moss effect. Burstein–Moss effect suggests bandgap energy broadening upon increase of Fermi level in the conduction band due to the excessive carriers from the doped metal cations (Hussain et al. 2007). Blueshift was observed independent of the concentration of the dopant ion. In addition to the ultraviolet (UV) emission, a shoulder peak centered at around 410 nm without any other emissions in the visible region was detected for all of the films. Visible emissions in ZnO are usually associated with the defects in the material which provide a trap for the photogenerated hole and prevent its recombination with the excited electron. Each of the emissions at different regions of the visible spectrum is attributed to discrete defects including singly ionized oxygen vacancy V +O , V 2+O center, oxygen antisite, interstitial oxygen defects Oi, excess oxygen, zinc vacancy VZn, and interstitial zinc Zni with a high degree of controversy (Tam et al. 2006). The violet PL band was reported to be originating from the transitions between the holes in the valence band and the electrons at the interstitial zinc, Zni level for ZnO film grown on quartz glass (424 nm) (Fan et al. 2006) as well as for ZnO nanorings (410 nm) prepared by a sol–gel route (Mondal and Pal 2011). Jin et al. also reported a violet emission centered at 420 nm (2.95 eV) for ZnO film on sapphire prepared by pulsed laser deposition (Jin et al. 2000). They attributed this violet emission to the transitions between the valence band and the interfacial traps that are present at the ZnO–ZnO grain boundaries. The interfacial trap explanation was also advocated by Wang et al. for the violet luminescence (402 nm) of ZnO films on Si (Wang et al. 2002). In line with the above discussion, Cui et al. ascribed the violet emission of ZnO film obtained by electrodeposition in DMSO:H2O mixture (419 nm) to the ZnO(DMSO)–ZnO(water) interface (Cui et al. 2011). Also in this study, most likely ZnO(DMSO)–ZnO(water) interface is responsible for the emission at 410 nm, since all of the doped films and the undoped ZnO exhibit the same transition. It is seen in Fig. 9 that the intensity ratio of the violet band to the UV band is the smallest for pure ZnO film and increases to the highest value for ZnO: Co films. The higher violet luminescence intensity suggests that the undoped film has smaller grains and larger grain boundary area than the ZnO: Co films which can be confirmed by SEM images and XRD calculations. Furthermore, the FWHM value of the PL peak of pure ZnO is higher than that of ZnO: Co films. The increased FWHM and lower intensity ratio of NBE to defect-related emission of the undoped ZnO film imply higher crystal quality for ZnO: Co films.
Conclusions
In summary, we report seedless hydrothermal–electrochemical growth of heterogeneous ZnO: Co nanostructures in DMSO:H2O mixture. Introduction of Co brought about a unique structure. Phase separation was observed together with the formation of Co-rich spheres on a zinc-rich matrix. The amount of Co determined the degree of phase separation and the quantity of the initial Co-rich spheres. XRD and XPS analyses indicated the presence of cobalt metal as well as a mixture of cobalt(II) and cobalt(III) oxides in cobalt-rich microzones. In view of this distinct morphology and structure, magnetization measurements on these films might help to answer the quest to find the presence and the origin of ferromagnetism in ZnO: Co films.
References
Bohle DS, Spina CJ (2010) Controlled Co(II) doping of zinc oxide nanocrystals. J Phys Chem C 114:18139–18145
Capitaneo JL, Simao RA, Guimarães AP, da Caffarena VR (2006) Preparation of electrodeposited cobalt nanowires. Mater Res 9:205–208
Chang YQ, Wang PW, Ni SL et al (2012) Influence of Co content on raman and photoluminescence spectra of Co Doped ZnO nanowires. J Mater Sci Technol 28:313–316
Cui H, Mollar M, Marí B (2011) Tailoring the morphology of electrodeposited ZnO and its photoluminescence properties. Opt Mater (Amst) 33:327–331
Cuscó R, Alarcón-Lladó E, Ibáñez J et al (2007) Temperature dependence of Raman scattering in ZnO. Phys Rev B 75:165202
Elbaum R, Vega S, Hodes G (2001) Preparation and surface structure of nanocrystalline cadmium sulfide (sulfoselenide) precipitated from dimethyl sulfoxide solutions. Chem Mater 13:2272–2280
Fan XM, Lian JS, Guo ZX et al (2006) Influence of the annealing temperature on violet emission of ZnO films obtained by oxidation of Zn film on quartz glass. J Mater Sci 41:2237–2241
Gupta V, Bhattacharya P, Yuzuk YI et al (2006) Optical phonon modes in ZnO nanorods on Si prepared by pulsed laser deposition. J Cryst Growth 287:39–43
Hagelin-weaver HAE, Hoflund GB, Minahan DM, Salaita GN (2004) Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar+ bombardment. Appl Surf Sci 235:420–448
Hsieh PT, Chen YC, Kao KS, Wang CM (2008) Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl Phys A 90:317–321
Hussain SG, Liu D, Huang X et al (2007) Synthesis and optical properties of partially S-doped ZnO symmetric three-sided feather-like nanostructures. Smart Mater Struct 16:1736–1741
Ivill M, Pearton SJ, Rawal S et al (2008) Structure and magnetism of cobalt-doped ZnO thin films. New J Phys 10:65002
Jiang J, Li L (2007) Synthesis of sphere-like Co3O4 nanocrystals via a simple polyol route. Mater Lett 61:4894–4896
Jiménez VM, Espinós JP, González-Elipe AR (1998) Control of the stoichiometry in the deposition of cobalt oxides on SiO2. Surf Interface Anal 26:62–71
Jin BJ, Im S, Lee SY (2000) Violet and UV luminescence emitted from ZnO thin films grown on sapphire by pulsed laser deposition. Thin Solid Films 366:107–110
Jug K, Tikhomirov VA (2009) Comparative studies of cation doping of ZnO with Mn, Fe, and Co. J Phys Chem A 113:11651–11655
Kim KS (1975) X-ray-photoelectron spectroscopic studies of the electronic structure of CoO. Phys Rev B 11:2177–2184
Lawes G, Risbud AS, Ramirez AP, Seshadri R (2005) Absence of ferromagnetism in Co and Mn substituted polycrystalline ZnO. Phys Rev B 71:45201
Li Y-J, Wang C-Y, Lu M-Y et al (2008) Electrodeposited hexagonal ringlike superstructures composed of hexagonal Co-doped ZnO nanorods with optical tuning and high-temperature ferromagnetic properties. Cryst Growth Des 8:2598–2602
Li T, Cao Z, You H et al (2013) Controllable growth of ZnO mesocrystals using a facile electrochemical approach. Chem Phys Lett 555:154–158. doi:10.1016/j.cplett.2012.10.046
Liu T, Xu H, Chin WS et al (2008) Local structures of Zn1−x TM x O (TM) Co, Mn, and Cu) nanoparticles studied by X-ray absorption fine structure spectroscopy and multiple scattering calculations. J Phys Chem C 112:13410–13418
Look DC (2001) Recent advances in ZnO materials and devices. Mater Sci Eng B 80:383–387
Lu X, Wang D, Li G et al (2009) Controllable electrochemical synthesis of hierarchical ZnO nanostructures on FTO glass. J Phys Chem C 2:13574–13582
Matei E, Enculescu I, Vasilache V, Teodorescu CM (2010) Cobalt-doped ZnO prepared by electrochemistry: chemistry, morphology, and magnetism. Phys Status Solidi 207:2517–2522
Mclntyre NS, Cook MG (1975) X-Ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal Chem 47:2208–2213
Milekhin AG, Yeryukov NA, Sveshnikova LL et al (2012) Surface enhanced Raman scattering of light by ZnO nanostructures. J Exp Theor Phys 113:983–991
Mondal O, Pal M (2011) Strong and unusual violet-blue emission in ring shaped ZnO nanocrystals. J Mater Chem 21:18354
Ohtomo A, Kawasaki M, Koida T et al (1998) Mg[sub x]Zn[sub 1 − x]O as a II–VI widegap semiconductor alloy. Appl Phys Lett 72:2466
Otani S, Katayama J, Umemoto H, Matsuoka M (2006) Effect of bath temperature on the electrodeposition mechanism of zinc oxide film from zinc nitrate solution. J Electrochem Soc 153:C551–C556
Ou Y-N, Liz G-R, Wang Z-L et al (2010) Electrodeposition and characterization of CoO/ZnO composite dendritic structures consisted of hexagonal nanoplatelets. J Electrochem Soc 157:D264–D268
Ozgur U, Alivov YI, Liu C et al (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:41301
Pan S, An Z, Zhang J, Song G (2010) Synthesis and hierarchical assembly of CoNi flowery particles. Mater Chem Phys 124:342–346
Panigrahy B, Aslam M, Bahadur D (2010) Aqueous synthesis of Mn- and Co-doped ZnO nanorods. J Phys Chem C 114:11758–11763
Park SK, Park JH, Ko KY et al (2009) Hydrothermal–electrochemical synthesis of ZnO nanorods. Cryst Growth Des 9:3615–3620. doi:10.1021/cg9003593
Park HK, Hong SP, Do YR (2012) Vertical growth of ZnO nanorods prepared on an ITO-coated glass substrate by hydrothermal–electrochemical deposition. J Electrochem Soc 159:D355. doi:10.1149/2.078206jes
Pauporte T, Cortes R, Froment M et al (2002) Electrocrystallization of epitaxial zinc oxide onto gallium nitride. Chem Mater 14:4702–4708
Pradhan D, Leung KT (2008) Controlled growth of two-dimensional and one-dimensional ZnO nanostructures on indium tin oxide coated glass by direct electrodeposition. Langmuir 24:9707–9716
Rodríguez-Gattorno G, Santiago-Jacinto P, Rendon-Vázquez L et al (2003) Novel synthesis pathway of ZnO nanoparticles from the spontaneous hydrolysis of zinc carboxylate salts. J Phys Chem B 107:12597–12604
Santamaria M, Asaro L, Bocchetta P et al (2013) Anodic electro deposition of CeO2 and Co-doped CeO2 thin films. J Electrochem Soc 160:D212–D217. doi:10.1149/2.065306jes
Schmidt-Mende L, Macmanus-Driscoll JL (2007) ZnO-nanostructures, defects, and devices. Mater Today 10:40–48
Serrano J, Widulle F, Romero AH et al (2003) Dependence of phonon widths on pressure and isotopic mass: ZnO. Pyhsica Status Solidi (b) 266:260–266
Sesha V, Shetty A, Dawson P et al (2012) Solution-based synthesis of cobalt-doped ZnO thin films. Thin Solid Films 524:137–143
Song C, Zeng F, Geng KW et al (2007) The magnetic properties of Co-doped ZnO diluted magnetic insulator films prepared by direct current reactive magnetron co-sputtering. J Magn Magn Mater 309:25–30
Tam KH, Cheung CK, Leung YH et al (2006) Defects in ZnO nanorods prepared by a hydrothermal method. J Phys Chem B 110:20865–20871
Thakur JS, Auner GW, Naik VM et al (2007) Raman scattering studies of magnetic Co-doped ZnO thin films. J Appl Phys 102:93904
Tortosa M, Mollar M, Mari B (2007) Synthesis of ZnCdO thin films by electrodeposition. J Cryst Growth 304:97–102
Tortosa M, Mollar M, Marí B (2008) Synthesis and structural studies of diluted magnetic semiconductors by electrodeposition. Phys Status Solidi 5:3467–3470
Tuan A, Bryan J, Pakhomov A et al (2004) Epitaxial growth and properties of cobalt-doped ZnO on α-Al2O3 single-crystal substrates. Phys Rev B 70:1–9
Wang QP, Zhang DH, Xue ZY, Hao XT (2002) Violet luminescence emitted from ZnO films deposited on Si substrate by rf magnetron sputtering. Appl Surf Sci 201:123–128
Wang X, Xu J, Yu X et al (2007) Structural evidence of secondary phase segregation from the Raman vibrational modes in Zn[sub 1−x]Co[sub x]O (0 < x<0.6). Appl Phys Lett 91:31908
Xia XH, Tu JP, Zhang J et al (2010) Cobalt oxide ordered bowl-like array films prepared by electrodeposition through monolayer polystyrene sphere template and electrochromic properties. ACS Appl Mater Interface 2:186–192
Xu S, Wang ZL (2011) One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Res 4:1013–1098
Yang S, Man BY, Liu M et al (2010) Structural, optical and magnetic properties of Zn1−x Co x O dilute magnetic semiconductors thin films by pulsed laser deposition. Phys B Condens Matter 405:4027–4031
Yao KX, Zeng HC (2009) Architectural processes and physicochemical properties of CoO/ZnO and Zn1−x Co x O/Co1−y Zn y O nanocomposites. J Phys Chem C 113:1373–1385
Yilmaz C, Unal U (2013) Electrochemical deposition of Mn: ZnO films under hydrothermal conditions. J Electrochem Soc 160:D163–D167
Yilmaz C, Unal U (2014) Synthesis and characterization of hierarchical ZnO structures by a single-step electrodeposition under hydrothermal conditions. Electrochim Acta 123:405–411
Yilmaz C, Unal U (2015) Single step synthesis of (α-Fe2O3) hematite films by hydrothermal electrochemical deposition. RSC Adv 5:16082–16088. doi:10.1039/C4RA16028B
Yilmaz C, Unal U (2016) Morphology and crystal structure control of α-Fe2O3 films by hydrothermal–electrochemical deposition in the presence of Ce3+ and/or acetate. RSC Adv. doi:10.1039/c5ra20105e
Yin Z, Chen N, Chai C, Yang F (2004) Structural and magnetic properties of insulating Zn1− x Co x O thin films. J Appl Phys 96:5093–5096
Yu H, Yu J, Liu S, Mann S (2007) Template-free hydrothermal synthesis of CuO/Cu2O composite hollow microspheres. Chem Mater 19:4327–4334
Zhai T, Xie S, Zhao Y et al (2012) Controllable synthesis of hierarchical ZnO nanodisks for highly photocatalytic activity. CrystEngComm 14:1850
Zhang J (2002) Control of ZnO morphology via a simple solution route. Chem Mater 14:4172–4177
Zhang XL, Qiao R, Kim JC, Kang YS (2008) Inorganic cluster synthesis and characterization of transition-metal-doped ZnO hollow spheres. Cryst Growth Des 8:2609–2613
Zhang C, Wang Y, Bi S, Luo G (2011) Preparation of mesoporous ZnO microspheres through a membrane-dispersion microstructured reactor and a hydrothermal treatment. Ind Eng Chem Res 50:13355–13361
Zhou H, Chen L, Malik V et al (2007) Raman studies of ZnO: Co thin films. Phys Status Solidi 204:112–117
Acknowledgements
The authors thank the Koc University Faculty of Science for their financial support. The authors also thank the Turkish Ministry of Development for the financial support provided for the establishment of the Koc University Surface Science and Technology Center (KUYTAM).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yilmaz, C., Unal, U. Hydrothermal–electrochemical growth of heterogeneous ZnO: Co films. Appl Nanosci 7, 343–354 (2017). https://doi.org/10.1007/s13204-017-0579-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0579-6