Abstract
Eco-friendly silver nanoparticles (AgNPs) have various applications in modern biotechnology for better outcomes and benefits to the society. In the present study, we report an eco-friendly synthesis of silver nanoparticles using Saraca indica leaf extract. Characterization of S. indica silver nanoparticles (SAgNPs) was carried out by Fourier transform infrared spectroscopy, scanning electron microscopy, energy dispersive spectrometry, Zeta potential, and transmission electron microscopy. SAgNPs showed antimicrobial activity against Gram-negative and Gram-positive bacteria.
Similar content being viewed by others
Introduction
Nanobiotechnology is the newest and one of the most promising areas of research in modern medical science (Murugan et al. 2012). Nanoparticles, which are made from comparatively larger bulk material exhibit new and improved properties depending on their relative size, distribution and morphology (Li et al. 2012). Nanoparticles present a higher surface to volume ratio with decreasing size (Singh et al. 2010). The biological effectiveness and value of nanoparticles increase proportionally with an increase in the specific surface area which is due to the increase in their surface energy and catalytic reactivity and also due to changes in physical, mechanical, optical and electromagnetic properties (Pradeep and Anshup 2009; Choi et al. 2007; Reddy et al. 2008; Okuda et al. 2005; Lala et al. 2007). Nanoparticles are synthesized using different methods and more routinely used chemical methods (Sundarrajan et al. 2010; Thakkar et al. 2010). However, chemical methods cannot avoid the use of toxic chemicals in the synthesis protocol. Hence, the need of the hour is to develop high-yielding, low-cost, non-toxic and environmentally friendly procedures. Therefore, the biological approach for the synthesis of nanoparticles becomes imperative (Parashar et al. 2009). Nanoparticles derived from gold, silver and platinum have medical as well as pharmaceutical applications. Therefore, there is a growing need to develop eco-friendly processes for nanoparticles synthesis without using toxic chemicals. The use of silver in combating ever increasing multi-drug resistant strains is evident since it has been recognized as an effective antimicrobial agent that exhibits low toxicity in humans and has diverse in vitro and in vivo applications (Siulvaenrg et al. 2007).
Synthesis of nanoparticles from plants is rapid, low-cost, eco-friendly, and a single step method (Inbakandan et al. 2010). The green synthesis of nanoparticles using biological extracts is very rapid and cost effective (Ankamwar et al. 2005).
The present study focuses on green synthesis of nanoparticles using Saraca indica leaf extract. S. indica, also known as ashoka, is a tree normally found in north India. It has got wide applications like use in ayurvedic medicines to cure gynaecological disorders like pelvic pain, endometriosis (Rathee et al. 2010), menorrhagia, uterine fibroids, (Perugu et al. 2012) haemorrhagic dysentery (Jiang et al. 2009). The medicinal property of Saraca is attributed to ketosteroids, flavonoids, and phenolic and aldehydes compounds present in leaves, bark and stem. Considering the variety of uses and applications and little information on the synthesis of silver particle using leaf extract of S. indica, we have undertaken this study to synthesize and characterize silver nanoparticles and also to investigate the antibacterial activities of the synthesized nanoparticles.
Materials and methods
Silver nitrate (AgNO3) was procured from Merck, Mumbai, India. S. indica leaves were collected from Botanical garden, Erragadda, Hyderabad, India. Bacterial strains Gram-negative Escherichia coli and Pseudomonas aeruginosa and Gram-positive bacteria Staphylococcus aureus and Pseudomonas putida were procured from institute of microbial technology Chandigarh, India.
Preparation of plant leaf extract
The fresh and healthy leaves of the S. indica were collected 25 g of leaves were weighed and thoroughly washed with distilled water thrice and followed by Millipore water to remove the dust particles and other contaminants from the leaves (Farooqui et al. 2010; Nagati et al. 2012). The leaves were chopped into small pieces, to which 100 ml of Millipore water is added in a fresh conical flask and the mixture is boiled for 15 min. The plant extract was filtered using Whatman no. 1 filter paper and was stored at 4 °C for further studies.
Preparation of 1 mM silver nitrate (AgNO3) solution
For the preparation of 1 mM AgNO3, we took 0.0421 g of AgNO3 in 100 ml of Millipore water mixed thoroughly and filtered through Whatman no. 1 filter paper and placed in dark room.
Synthesis of silver nanoparticles
10 ml of S. indica leaf extract was mixed with 90 ml of 1 mM silver nitrate and heated on hot plate @60c for 30 min the colour of the mixture changed from light greenish yellow to dark brown which indicates the formation of silver nanoparticles (Fig. 1). The reduction of Ag+ was analysed by measuring the UV–Vis spectra at the range of 200–800 nm at every 5 min time intervals.
Characterization of silver nanoparticles
UV–visible spectroscopy
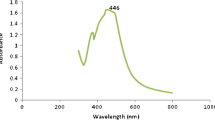
The formation of AgNps in the polymeric media was further determined using the UV–visible spectroscopy, a well-defined absorption peak at 450 nm was exhibited by the nano metallic silver particles and consequent colour changes from greenish yellow to reddish brown confirm the successful synthesis of silver nanoparticles, (leaf extract was used as blank). The absorption spectrums of the silver nanoparticles with 5 min time interval are shown in Fig. 2. The concentration of silver nanoparticles (AgNps) increased as the time increased.
Fourier transform infrared spectroscopy (FTIR)
The chemical components were observed on Bruker Optics, Tensor 27 Instrument. The IR spectrum of the silver nanoparticles is shown in Fig. 3. The IR spectrum revealed a characteristic peak at 1600–1800 cm−1 which corresponds to carbonyl group, flavonoids and steroids (Dubey et al. 2010). Hence, those were responsible for reduction and efficient stabilization. Results indicate that flavonoids and steroids in the leaf extract are involved in the reduction and stabilization of silver nano particles.
Zeta (potential) sizer
The zeta potential of the synthesized silver nanoparticles was determined in water as dispersant (Shameli et al. 2013). Laser diffraction revealed that particles obtained are polydisperse mixture with the size ranging from 10 to 400 nm (Fig. 4), the average diameter of the particles was found to be 98 nm, the zeta potential was found to be −55.0 mv. The high negative value confirms the repulsion among the particles and the negative value also indicates that nanoparticles are stable. The size and zeta potential of silver nanoparticles samples dispersed in different solutions were characterized by Jacobasch et al. 2012. The effects of ionic strength and pH on the state of dispersion were studied using titanium dioxide nanoparticles as a model (Raut Rajesh et al. 2009).
Scanning electron microscope (SEM) analysis
Scanning Electron Microscope analysis was performed using ZIESS (Evo-18), Tuscan VEGA II LSU electron microscope (Tuscan USA Inc.). Conditions used were: extra high voltage 10 kV, working distance 7.0 mm, display mode secondary electrons, high vacuum, and room temperature (30 °C). The polydispersed silver nanoparticles were mostly spherical in shape as shown in Fig. 5.
Energy dispersive spectrometry (EDS)
Energy dispersive (ED) (Inca X-act) showed the ED spectrum of the synthesized silver nanoparticles. Strong silver signal along with a weak oxygen, and silicon peak was observed.
TEM analysis
Transmission electron microscope (TEM) analysis was performed on a JEOL JEM-2010 (HT) electron microscope, using an accelerating voltage of 200 kV. The samples were dissolved in deionized water solution with concentrations of 0.5 mg ml−1, and a drop was placed on Cu grids precoated with carbon films. TEM technique was employed to visualize the size and shape of silver nanoparticles.
Antibacterial activity
Antibacterial activity of rapid biological synthesized S. indica silver nanoparticles was analysed against Gram-negative (E. coli (MTCC1303) and P. putida (CT2440)) and Gram-positive (S. aureus (CCMB263) and Micrococcus luteus (MTCC2987)) bacteria. The zone of inhibition of silver nanoparticles, against Gram-negative and Gram-positive bacteria was evaluated by the disc diffusion method (Morones et al. 2005; Perez et al. 1990; Shameli et al. 2012). Sterile Whatman no. 1 filter paper discs were placed on spread culture of LB agar. 5 µl of ampicillin (1 mg ml−1) was used as a positive control, 10 µl of S. indica AgNPs and 10 µl of S. indica extract were carefully placed on discs and incubated for 24 h at 37 °C.
The inhibition of bacteria was appeared as a clear transparent area around the discs. Those are called inhibition zones, such zone of inhibition was measured using a metre ruler and the mean value for each organism was recorded and expressed in millimetre (Table 1).
Results and discussion
This observation corroborated with results of UV–Vis spectral analysis. A well-defined absorption peak at 450 nm in UV–Vis spectral analysis indicates the nano metallic Ag particles, and consequent colour change from greenish yellow to reddish brown confirms the successful synthesis of silver nanoparticles. Peak shifts were taken at 0–25 min, with 5 min time intervals, there is gradual increase in the formation of AgNps with time interval as shown in Fig. 2.
The FTIR measurements of biosynthesized silver nanoparticles were carried out to identify the interaction between bio-organics of leaf extract and nanoparticles. FTIR spectra of silver nanoparticles showed absorption peak positioned at about 3397, 2809 (Hydrogen bond OH stretch), 1632 (C=C), 1386 (CH3 and CH2 deformation), 1095 (C–C), and 800 cm−1 (Fig. 3). The very intense broadband located at around 3397 and 2964 cm−1 position in the spectra of silver nanoparticles corresponds to the Alcohol/Phenol O–H Stretch and Carboxylic Acid O–H stretching. The stretch located at around 1621 cm−1 in the spectra of AgNPs represented C–C=C symmetric stretching of alkenes group while stretching vibration at 2856 cm−1 in the spectrum represents C–H stretching of aldehydes group.
Typical SEM images of silver nanoparticles synthesized in the present work are presented in Fig. 5 with different magnification scale. The biosynthesized silver nanoparticles were in mostly spherical in shape. The measured sizes of the agglomerated nanoparticles were in the range 51–230 nm, with poly dispersions. To confirm the chemical composition, energy dispersive X-ray analysis (EDAX) was acquired, and is shown in Fig. 6. We observed the existence of Ag, Si and intense O in the samples.
Transmission electron microscope (TEM) images and their corresponding particle size distributions of AgNPs at 25 min of time are shown in Fig. 7. The size and shape of silver nanoparticles are clearly observed. It is very clear that the silver nanoparticles were distinct and spherical in shape, with an average particle size of 23 ± 2 nm.
Gram-positive bacteria had a thick cell wall, containing a high amount of peptidoglycan and Gram-negative bacteria had two layers of cell membrane: inner membrane contains peptidoglycan and the outer membrane contains lipopolysaccharides. The results indicated that silver nanoparticles synthesized from S. indica extract showed effective antibacterial activity both against Gram-negative and Gram-positive bacteria (Fig. 8).
Conclusion
Green synthesis of silver nanoparticles is desirable over other methods of synthesizing nanoparticles as it is widely applied in the field of nanotechnology and nanobiotechnology (nanomedicine). This study focuses on synthesizing nanoparticles using S. indica leaf extract. The synthesized nanoparticles were characterized by different biophysical methods such as FTIR, UV–Vis Spectrophotometer, Zeta sizer, SEM, EDS, and TEM. The findings suggest that nanoparticles are spherical in shape with 23 ± 2 nm size. Further, the green synthesis of silver nanoparticles using leaf extract of medicinally potent plant S. indica showed potential antibacterial activity against both Gram-positive and Gram-negative strains.
References
Ankamwar B, Damle C, Ahmed A, Sastry M (2005) Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5:1665–1671
Choi S, Kim KS, Yeon SH, Cha JH, Lee H, Kim CJ, Yoo ID (2007) Fabrication of silver nanoparticles via self-regulated reduction by 1-(2-hydroxyethyl)-3-methylimidazolium tetrafluoroborate. Korean J Chem Eng 24(5):856–859
Dubey SP, Lahtinen M, Sillanpaa M (2010) Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp 364:34–41
Farooqui MDA, Chauhan PS, Krishnamoorthy P, Shaik J (2010) Extraction of silver nanoparticles from the leaf extracts of Clerodendrum Inerme. Dig J Nanomater Biostruct 5(1):43–49
Inbakandan D, Venkatesan R, Khan SA (2010) Biosynthesis of gold nanoparticles utilizing marine sponge Acanthella elongata (Dendy, 1905). Colloids Surf B Biointerfaces 81(2):634–639
Jacobasch HJ, Simon F, Werner C, Bellmann C (2012) Determination of the zeta potential from streaming potential and streaming current measurements. Tech Mess 63(12):447–452
Jiang J, Oberdorster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89
Lala NL, Ramakrishnan R, Bojun L, Sundarrajan S, Barhate RS, Ying-jun L, Ramakrishna S (2007) Fabrication of nanofibers with antimicrobial functionality used as filters: protection against bacterial contaminants. Biotechnol Bioeng 97(6):1357–1365
Li Y, Wu T-Y, Chen S-M, Ali MA, AlHemaid FMA (2012) Green synthesis and electrochemical characterizations of gold nanoparticles using leaf extract of Magnolia kobus. Int J Electrochem Sci 71:2742–12751
Morones JR, Elechiguerra JL, Camacho A (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Natesan M, Chauhan RC, Cherian J, Purty AC, Singh Z, Joice S, Abraham SB (2015) Patient and health system delay among new pulmonary tuberculosis patients diagnosed at medical college hospitals in Puducherry, India. Int J Res Med Sci 3(1):188–193
Nagati V, Koyyati R, Donda MR, Alwala J, Kundle KR, Padigya PRM (2012) Green Synthesis and characterization of Silver nanoparticles from Cajanus cajan leaf extract and its antibacterial activity. Int J Nanomater Biostruct 2:39–43
Okuda M, Kobayashi Y, Suzuki K, Sonoda K, Kondoh T, Wagawa A, Kondo A, Yoshimura H (2005) Self-organized inorganic nanoparticle arrays on protein lattices. Nano Lett 5:991–993
Parashar UK, Saxena SP, Srivastava A (2009) Bioinspired synthesis of silver nanoparticles. Dig J Nanomater Biostruct 4(1):159–166
Perez C, Paul M, Bazerque P (1990) An antibiotic assay by the agar well diffusion method. Acta Biol et Med Exp 15:113–115
Perugu S, Bade PN, Nawaz SS (2012) ECDB: a database for endometrial cancer. J Comput Intell Bioinform 5:161–167
Pradeep T, Anshup (2009) Noble metal nanoparticles for water purification: a critical review, invited critical review. Thin Solid Films 517:6441–6478
Rathee P, Rathee S, Rathee D, Rathee D (2010) Quantitative estimation of (+) Catechin in stem bark of Saraca asoka Linn using HPTLC. Der Pharma Chem 2:306–314
Raut Rajesh W, Lakkakula Jaya R, Kolekar Niranjan S, Mendhulkar Vijay D, Kashid Sahebrao B (2009) Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr Nanosci 5(1):117–122
Reddy KR, Lee K-P, Lee Y, Gopalan AI (2008) Facile synthesis of conducting polymer–metal hybrid nanocomposite by in situ chemical oxidative polymerization with negatively charged metal nanoparticles. Mater Lett 62(12–13):1815–1818
Shameli K, Ahmad MB, Jazayeri SD, Shabanzadeh P, Sangpour P, Jahangirian H, Gharayebi Y (2012) Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem Cent J 6(73):1–10
Shameli K, Ahmad MB, Shabanzadeh P, Al-Mulla EAJ, Zamanian A, Abdollahi Y, Jazayeri SD, Eili M, Jalilian FA (2013) Effect of Curcuma longa tuber powder extract on size of silver nanoparticles prepared by green method. Res Chem Intermed. doi:10.1007/11164-013-1040-4
Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma DHN (2010) Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. J Nanomat Biostruct 5:483–489
Siulvaenrg H, Nuann Do A, Oq D, Alrut Iycl DSP, Yoavnelg Sxu N (2007) Bcioinsynnatmheosmisu Mofcamphora leaf. Nanotechnology 18:103–105
Sundarrajan S, Chandrasekaran AR, Ramakrishna S (2010) An update on nanomaterials-based textiles for protection and decontamination. J Am Ceram Soc 93(12):3955–3975
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanobiotechnol Biol Med 6(2):257–262
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Funding
This study was supported by in part by grants from UGC, India (No: 23/23/UGC/UPE/FAR/OU/2014).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Perugu, S., Nagati, V. & Bhanoori, M. Green synthesis of silver nanoparticles using leaf extract of medicinally potent plant Saraca indica: a novel study. Appl Nanosci 6, 747–753 (2016). https://doi.org/10.1007/s13204-015-0486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-015-0486-7