Abstract
Reaction variables for methanation process were investigated using self-independently developed NiO–La2O3–MgO/Al2O3 catalyst in a big lab scale reactor. The effects of reaction parameters, such as temperature, pressure, H2/CO flow ratio and space velocity on the activity of methanation catalyst were studied. 100 % CO conversion and 95 % of selectivity of methane can be achieved at 400 °C and 3 MPa with the feed ratio of H2/CO as 3.25:1 and space velocity of 12,000 h−1.The optimization reaction parameters were suggested on the basis of this work for the further development and commercialization of methanation catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methanation is one of the key techniques for the coal to synthetic natural gas [1]. This technology requires catalyst with high activity and high stability due to the strong exothermicity of the methanation reaction. Currently, commercial methanation technology is being supplied by Lurgi (LURGI), Johnson Matthey (DAVY) and Topsoe (TOPSØE). All of these processes are equipped with a fixed-bed reactor [2–4]. Ni-based catalyst is often the most practical choice for methanation because of its relatively high activity and low price. NiO/Al2O3 catalysts have been intensively explored for the methanation of carbon monoxide [5].
A satisfactory methanation catalyst should be excellent, coking-resistant, and possess high thermal stability at high temperature and pressure [6–8]. The target of this work is to develop a nickel-based catalyst. It is found that the addition of lanthanum increased the catalyst stability [9]. MgO was found to be an effective promoter to improve resistance to carbon deposition and to minimize Ni particles’ sintering [10–12]. In this paper, we would like to study the effect of the reaction conditions on the NiO–La2O3–MgO/Al2O3 catalyst to investigate the effects of reaction variables on the methanation. Moreover, the operation parameters would be optimized for the further development and commercialization of methanation catalyst.
Experimental method
Experimental device
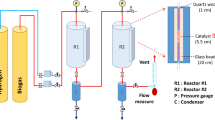
All the experiments were carried out with a high-temperature, high-pressure fixed-bed reactor system. The flow chart of the experimental apparatus is shown in Fig. 1. The system is composed of three parts, which are gas mixer system, reactor system and analysis system. The feed gas is decompressed by the pressure-reducing valves of different gas cylinders. The flow rate of feed gas is controlled by a mass flowmeter. After mixing, proportional synthesis gas is introduced into the high-pressure reactor. The tail gas from the reactor is cooled with 10 °C cold water trap to convert it into gas and liquid phases. Then the outlet gas filtered by dryer is connected to an Agilent GC 7890B instrument for online measurement. The liquid product collected from the liquid collector was weighed and analyzed afterward. During the reaction, the content of all components was analyzed by GC 7890B, such as CO, CO2, H2, CH4, C2H6 and C3H8 by TCD and FID detectors with proper column separation. Carbon monoxide conversion (C CO), methane space–time yield (STYCH4) and selectivity to methane (S m) were determined by following formulas:
mcat: Mass of the catalyst (g); V i,in (ml/min) and V i,out (ml/min) are the inlet and outlet volumetric flow rate of species i (i = CO, CH4), respectively.
Catalyst preparation
The NiO–La2O3–MgO/Al2O3 catalyst precursor was prepared using coprecipitation method at constant pH. Aqueous solutions of the metal nitrates and the precipitating agent (NaOH/Na2CO3) were simultaneously dripped into beaker. The pH during precipitation in the stirred beaker was maintained at a constant value 8. After finishing the addition of the precipitation agent, the resultant slurry was aged at 80 °C for 12 h under constant stirring, followed by filtration, washing and drying overnight at 120 °C. The catalyst precursor was calcined in a muffle burner at 450 °C for 4 h. Graphite and cement were added in the catalyst precursor for granulation and tableting, steam and curing treatment. The resultant catalyst products have NiO content of 40 wt %, MgO content of 15 wt % and La2O3 content of 5 wt %. It has relatively more meso-pores with average pore size of 9.5 nm. It was ground and meshed to 425–850 μm particles for experiments.
Catalyst evaluation
The catalyst sample was loaded into a pressurized fixed-bed reactor. Firstly, a gas stream consisting of H2:N2 with ratio of 1:4 was used for NiO reduction for 4 h at temperature of 550 °C. Then, the reactor was cooled to 200 °C at the end of the reduction. The pressure of the system was slowly increased with nitrogen gas. The feed gas was switched into the reactor gradually to the required pressure. The activity of methanation catalyst was tested for reaction temperature of 250–700 °C, the reaction pressure of 1–4 MPa, the flow ratio of the H2/CO as 2:1–4:1 and the space velocity of 6000–48,000 h−1.
Result and discussion
Reaction temperature
Holding the reactor pressure at 1 MPa, a space velocity of 12,000 h−1 and H2/CO of flow rate of 3, the effect of temperature on methanation reaction was investigated from 250 to 700 °C. The experiment results are shown in Fig. 2.
With increase of temperature, carbon monoxide and hydrogen conversion rate, selectivity and space–time yield of methane were firstly increased and then decreased gradually. When the reactor temperature reached 400 °C, the CO conversion and the H2 conversion reached the highest value. This is due to the methanation reaction being an exothermic reaction; lower temperature favors the CO conversion [13]. When the reaction temperature increased, the equilibrium constant of the reaction rate decreased, leading to a lower CO conversion. Moreover, the methanation reaction is a reversible reaction. Methanation reaction equilibrium moves in the reverse direction at higher temperature. All this would inhibit the conversion of CO at high temperature [14].
For methanation reaction, the basic requirements for the catalyst are that it must maintain the activity at the temperatures during the normal operation, meanwhile, it should be able to start up the reaction at the possible lowest temperature so as to keep the inlet temperature as low as possible. This would relieve the thermal effects of the entire reactor bed [15]. Under our experimental conditions, the optimal temperature range is between 350 and 450 °C; methanation reached the desired CO conversion between 350 and 450 °C temperature range, which is consistent with the thermodynamic prediction of methanation by previous researchers [16, 17].
Reaction pressure
Maintaining a temperature of 350–450 °C, a space velocity of 12,000 h−1, H2/CO ratio of flow rate of 3, the effect of pressure on methane synthesis reaction was studied from 1 to 4 MPa. The experiment results are shown in Figs. 3, 4 and 5.
Figures 3, 4 and 5 present the change of carbon monoxide and hydrogen conversion, methane selectivity and STY at different temperatures and different pressures. When the temperature was 350 °C, the conversion rate of carbon monoxide, hydrogen and methane increased gradually with the increase of pressure, while the selectivity of methane increased and then tended to be stable. The space–time yield of methane was gradually increased with the increase of pressure as well. The carbon monoxide conversion rate remained at 100 % as the pressure increased, when the temperature was 400–450 °C. However, the space–time yield of methane remained constant when the temperature was 400–450 °C. According to the kinetics of the reaction, the number of moles of carbon monoxide methanation reaction is reduced; increasing of pressure is in favor of the reaction equilibrium because the reaction constant is shifted to the gas volume decreasing direction [18, 19]. The experimental data showed that the conversion of carbon monoxide can be improved at a relatively low temperature with the increase of pressure. However, the increase of pressure has little effect on the conversion of carbon monoxide when temperature is higher than 350 °C. It indicated the high pressure was not necessary for methanation reaction pressure at high temperature. When the pressure increases to a certain degree, the catalyst activity is not affected. The optimal pressure must be determined when considering the load of industrial production and the thermodynamic effects of methanation reaction in the normal production process.
H2/CO ratio of the syngas
Maintaining a temperature of 400 °C, 1 MPa pressure, the effect of H2/CO ratio on methane synthesis reaction was studied at flow rate of 2:1, 2.5:1, 3:1, 3.25:1, 3.5:1, 3.75:1 and 4:1. The experiment results are shown in Fig. 6.
Figure 6 shows that the trends of methane selectivity and the space–time yield of carbon monoxide and hydrogen change with the H2/CO ratio. However, the carbon monoxide conversion rate tended to be stable at the H2/CO ratio of more than 3. The best theoretical molar ratio of the methane reaction is H2/CO flow ratio of 3:1, when the mass transfer process is not considered [20]. However, in order to avoid carbon deposition on the surface of nickel catalyst, the practical reaction condition of H2/CO ratio is usually higher than the stoichiometric ratio [21, 22]. The conversion rate of carbon monoxide and the space–time yield of methane were highest when the H2/CO ratio of the raw gas was 3.25:1, which was suggested as most suitable for the process of methane conversion.
Space velocity
Maintaining a temperature of 400 °C, 1 MPa pressure, H2/CO ratio of flow rate of 3.25:1, the effect of space velocity on methane synthesis reaction was studied at 6000, 12,000, 18,000, 24,000 and 48,000 h−1 conditions. The experiment results are shown in Fig. 7.
Figure 7 shows that the conversion of carbon monoxide and hydrogen, selectivity and the space–time yield of methane are increased with the increase of space velocity. The methanation reaction activity was gradually decreased when the velocity is higher than 12,000 h−1. Then, the space–time yield of methane downward trend is more significant. This is because the contact time between the feed gas and the catalyst surface becomes shorter at high space velocity. The effective collision between reactants and feed gas is reduced, and the conversion efficiency of the methanation reaction is reduced. Production at low space velocity is beneficial to improve single pass conversion of carbon monoxide in the actual production process, but productivity of the reactor will be very low. Single pass conversion rate was lower in high space velocity operating conditions. In order to improve the utilization efficiency of the feed gas, the feed gas needs many cycles, which will increase the operation cost. Thus, a proper space velocity needs to be selected to ensure that the catalyst activity is optimal in the actual production. Our experimental results suggest that the space velocity of 12,000 h−1 gives a balance of operation and productivity.
Conclusion
In this study, the effects of the reaction variables such as temperature, pressure, H2/CO ratio of the feed and space velocity on the activity of methanation catalyst for CO methanation have been investigated. According to the experimental results, the high pressure was not necessary for methanation reaction pressure at high temperature. The optimal pressure needs to be determined when considering the load of industrial production and the thermodynamic effects of methanation reaction in the normal production process. From our experimental, the optimal temperature range is between 350 and 500 °C, the optimal H2/CO ratio of the raw gas is 3.25:1 and the optimal space velocity is 12,000 h−1. These parameters were suggested as most suitable for the process of syngas to methane conversion.
References
Kopyscinski J, Schildhauer TJ, Biollaz SMA (2010) Production of synthetic natural gas (SNG) from coal and dry biomass—a technology review from 1950 to 2009. Fuel 89:1763–1783
Kustov AL, Frey AM, Larsen KE et al (2007) CO methanation over supported bimetallic Ni–Fe catalysts: from computational studies towards catalyst optimization. Appl Catal A Gen 320:98–104
Panagiotopoulou P, Kondarides DI, Verykios XE (2008) Selective methanation of CO over supported noble metal catalysts: effects of the nature of the metallic phase on catalytic performance. Appl Catal A Gen 344:45–54
Bligaard T, Nørskov JK, Dahl S et al (2004) The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J Catal 224:206–217
Huang Y, Chen H, Su J, Xiao T (2014) Highly active and selective catalyst for synthetic natural gas (SNG) production. Appl Petrochem Res 4:181–188
Kok E, Scott J, Cant N et al (2011) The impact of ruthenium, lanthanum and activation conditions on the methanation activity of alumina-supported cobalt catalysts. Catal Today 164:297–301
Frøseth V, Storsæter S, Borg Ø et al (2005) Steady state isotopic transient kinetic analysis (SSITKA) of CO hydrogenation on different Co catalysts. Appl Catal A Gen 289:10–15
Kimura M, Miyao T, Komori S et al (2010) Selective methanation of CO in hydrogen-rich gases involving large amounts of CO2 over Ru-modified Ni-Al mixed oxide catalysts. Appl Catal A Gen 379:182–187
Bang Y, Seo JG, Song IK (2011) Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni–La–Al2O3 aerogel catalysts: effect of La content. Int J Hydrog Energy 36:8307–8315
Cheekatamarla PK, Finnerty CM (2006) Reforming catalysts for hydrogen genenration in fuel cell application. J Powe Sources 160(1):490–499
Zhanga G, Suna T, Penga J et al (2013) A comparison of Ni/SiC and Ni/Al2O3 catalyzed total methanation for production of synthetic natural gas. Appl Catal A Gen 462–463:75–81
Romero A, Jobbágy M, Laborde M et al (2010) Ni(II)–Mg(II)–Al(III) catalysts for hydrogen production from ethanol steam reforming: influence of the activation treatments. Catal Today 149:407–412
Zhang H, Yunyun D, Weiping F et al (2013) Effects of composite oxide supports on catalytic performance of Ni-based catalysts for CO methanation. Chin J Catal 34:330–335
Panagiotopoulou P, Kondarides DI, Verykios XE (2009) Selective methanation of CO over supported Ru catalysts. Appl Catal B Environ 88:470–478
Lebarbier VM, Dagle RA et al (2014) Sorption-enhanced synthetic natural gas (SNG) production from syngas: a novel process combining CO methanation, water-gas shift, and CO2 capture. Appl Catal B Environ 144:223–232
Gao J, Wang Y, Ping Y et al (2012) A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv 2:2358–2368
Bang Y, Seo JG, Song IK (2011) Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni–La–Al2O3 aerogel catalysts: effect of La content. Int J Hydrog Energy 36:8307–8315
Kowalczyk Z, Stolecki K, Raróg-Pilecka W et al (2008) Supported ruthenium catalysts for selective methanation of carbon oxides at very low COx/H2 ratios. Appl Catal A Gen 342:35–39
Engbæk J, Lytken O, Nielsen JH et al (2008) CO dissociation on Ni: the effect of steps and of nickel carbonyl. Surf Sci 602:733–743
Yu Y, Jin G-Q, Wang Y-Y et al (2011) Synthetic natural gas from CO hydrogenation over silicon carbide supported nickel catalysts. Fuel Process Technol 92:2293–2298
Trimm DL (1999) Catalysts for the control of coking during steam reforming. Catal Today 49:3–10
Sehested J, Dahl S, Jacobsen J et al (2004) Methanation of CO over nickel: mechanism and kinetics at high H2/CO ratios. J Phys Chem B 109:2432–2438
Acknowledgments
This work presented in this paper was funded by China Huaneng Group (CERI/TY-14-HJK04). The authors would also like to thank Professor Shuren Hao for providing constructive advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
He, Z., Wang, X., Gao, S. et al. Effect of reaction variables on CO methanation process over NiO–La2O3–MgO/Al2O3 catalyst for coal to synthetic natural gas. Appl Petrochem Res 5, 413–417 (2015). https://doi.org/10.1007/s13203-015-0127-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-015-0127-9