Abstract
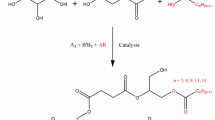
Diacyl amino acid surfactant was prepared using lauroyl chloride and l-lysine. The structure of the synthesized surfactant was confirmed by infrared spectroscopy and mass spectroscopy. The ultralow interfacial tension could be achieved when the diacyl amino acid surfactant is mixed with sulfonate in the presence of polymer as well as absence of polymer. Core flood experiments showed that 17.8 % additional oil could be recovered by injection of 0.3 pore volume of surfactant–polymer solution, making the total oil recovery reach 66.1 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactant is an important type of fine chemical product, which is often dubbed as “the industrial monosodium glutamate” and is consumed in large quantities [1]. However, the water and soils will be contaminated when a large amount of surfactants are discharged into them. In recent years, to meet requirement of the environmental protection and biological safety, more attention has been paid to develop and use the environment-friendly surfactants.
Amino acid-based surfactants are a class of surfactants with excellent biocompatibility, emulsifying ability, low toxicity, low irritation, as well as antimicrobial properties [2, 3]. They are now very commonly used in house cleaning products [4, 5], food industry, pharmaceutical applications, and foam flotation, textile and leather industry, but seldom in oil recovery.
It is well known that, the oil recovery can be improved by injecting surfactants, alkalis or polymers to displace the residual oil that trapped in the reservoir. Nevertheless, the alkali containing flooding can cause many problems, such as erosion, reduction of productivity and the shortening of pump checking [6, 7]. To avoid using alkali, we tend to develop alkali-free surfactant–polymer flooding system. One of the structural characteristics of the flooding using surfactants is branched. This is because branched surfactants often show a better interfacial tension lowering ability and effectiveness than those with straight-chain alkyl groups [8, 9]. Low interfacial tension is necessary to mobilize the trapped oil [10]. The conventional surfactants are often single tailed. Here we designed and prepared a double-chain amino acid surfactant diacyl amino acid sodium (DAAS), and evaluated its potential in surfactant–polymer flooding. It should be mentioned that, one of its chains could be seen as a side chain.
Experimental
Chemicals
Lauroyl chloride, sodium hydroxide, and acetone (AR grade) were purchased from West Asia chemical reagent Company. l-Lysine was purchased from Guangfu Fine Chemicals Research Institute. Hydrolyzed polyacrylamide (HPAM), degree of hydrolysis is 22.8 % with a molecular weight of 2.4 × 107, was obtained from Hengju company. Daqing petroleum sulfonate (DPS) was offered by Daqing refinery factory. It was washed and purified by washing with petroleum ether and ethanol to remove the unreacted oil and salts. The crude oil used in this experiment was extracted from Jing11 oil well. The density of the crude oil is 0.81 kg/m3, the water used in the experiment is the injection water, its salinity is 10,954 mg/L.
Synthesis
The amino acid surfactant was synthesized according to the [1].The synthetic route was presented in Scheme 1. Acetone (50 ml), water (25 ml) and l-lysine (7.3 g) were added to a 500 ml three-necked round-bottomed flask equipped with a mechanical stirrer. The mixture was stirred while 32.76 g lauroyl chloride and 10 % (wt) sodium hydroxide solution were added drop wise. Then, the mixture was vigorously stirred for 4 h in an ice bath while the pH was kept near 9, followed by overnight standing at room temperature. The mixture was placed under vacuum to remove the solvent, then the hydrochloric acid was added to adjust the pH value to 2. The obtained product was separated by sucking filtration and washing with deionized water until neutral. The product was then washed three times with petroleum ether and dried under vacuum. The obtained amino acid was dispersed in deionized water at 80 °C and neutralized with an equal molar quantity of sodium hydroxide (mass concentration was 10 %). The final product was purified by sucking filtration, drying and recrystallization with ethanol.
Characterization
The authors characterized the surfactant that had been synthesized in-house using IR and MS.
Infrared spectra were recorded as KBr pellets on a Nicolet 8700 FT-IR spectrometer in the scanning range of 4,000–500 cm−1. The KBr pellets were prepared by gently mixing 1 mg of the dried sample with 100 mg of the dried KBr. MS analysis was carried out on a Waters Quattro Premier XE in a positive mode with an m/z 50–1,000 scanning range using methanol as a mobile phase. The operating parameters were set as follows: capillary voltage, 3.5 kV; cone voltage, 30 V; source temperature, 120 °C; desolvation temperature, 150 °C; desolvation gas flow rate, 600 L/h. The sample solution was prepared by dissolving 10 mg of the sample in 10 mL of the mobile phase, then injected into the mass detector. Interfacial tensions were measured at 54 °C (reservoir temperature) using a TX550A spinning-drop tensiometer at a rotation speed of 5,000 rpm. The measurement time was 120 min. The sample was dissolved in the injection water, then injected into a sample tube using a syringe. Core flood test was performed on a sand-packed core of diameter 2.5 cm and length 50 cm. The permeability of the core was 248.6 mD with the pore volume of 74.6 ml. The porosity of the core was obtained by dividing the pore volume by the volume of the packed core. Its value was 30.41 %. The oil saturation was calculated by dividing the volume of the oil that was saturated into the core by the pore volume. Its value was 81.1 %.
Results and discussion
Infrared spectroscopy of DAAS
The infrared spectrum of DAAS is shown in Fig. 1. The bands at 3,324 and 1,421 cm−1 are due to N–H stretching vibrations. The band at 2,955 cm−1 is attributed to antisymmetric CH3 stretching vibrations while the band at 2,920 cm−1 can be assigned to antisymmetric CH2 stretching vibrations. The band observed at 2,850 cm−1 can be attributed to the symmetric CH2 stretching vibrations. The absorption at 1,641 cm−1 is due to the amide band whereas the band at 1,557 cm−1 can be assigned to C–N stretching and N–H bending vibrations.
ESI–MS spectra of DAAS
The positive ion ESI–MS spectra of DAAS are displayed in Fig. 2. The peak at m/z = 511.4 corresponds to [M−Na + H]+ where m/z = 512.4 corresponds to [M−Na + 2H]+. The minor peak at m/z = 329.2 signifies l-lysine is mono-substituted.
Interfacial tension behavior
Figure 3 shows the interfacial tension between Jing11 crude oil and injection water. It is observed that neither the DAAS nor the DPS could reduce the interfacial tension to ultralow values. It should be noted that, the DAAS can lower the interfacial tension to a magnitude of 10−2 mN/m at the concentration of 0.3 % (mass ratio). However, when DAAS was mixed with DPS, the ultralow interfacial tension could be reached in the absence of alkaline agents at a wide range of concentrations (see Table 1).
It is evident from Table 1 that the two surfactants showed the synergism in interfacial tension reduction. A possible reason may be that the interfacial activity of binary surfactant mixtures depends on their adsorption at the oil–water interface. And the adsorption is correlated with the tightness of mixed micellar structure [11]. For the DAAS and DPS molecules, their hydrophilic–lipophilic balance (HLB value, the DAAS has a value of 15.56, while for the conventional surfactants, the value is generally around 10) differences cause the different insertion depth at the oil/water interface, which has benefit of reducing repulsion between the polar groups [12] and then influences the tightness of the mixed micellar structure. The compact mixed micellar structures are easy to adsorb at the oil/water interface and have a relatively higher adsorption amount, so the interfacial activity is improved.
In the surfactant polymer flood process, the addition of water-soluble polymer can expand swept volume by increasing the water viscosity and decreasing water–oil mobility ratios [13]. However, it often has a negative effect on the interfacial tension. In view of the interfacial tension factors, the formulation (0.05 % DAAS + 0.1 % DPS) was chosen to investigate the effect of the polymer (HPAM) on the interfacial tension. The concentration of the polymer was 1,500 mg/L. The results are presented in Fig. 4.
Figure 4 reveals that the interfacial tension increased in the presence of polymer, but still lower than 10−2 mN/m. A possible reason for the interfacial increase is that there were hydrophobic domains existed in the polymer solution. The surfactant would adsorb in the hydrophobic domains when added into the solution and formed mixed micelles with hydrophobic chains [14]. Thus, the surfactant adsorption amount at the oil/water interface was cut, which resulted in the interfacial tension decrease. Besides, the reason for the sharp deviation of the mixed surfactant system at early times is: the oil droplet was first elongated, then broken up into several smaller droplets. The retraction of the droplet caused the interfacial tension increase.
Flooding experiments
The permeability of the major reservoirs is around 250 mD, so a sand-packed core with a permeability of 248.6 mD was prepared. The core was first evacuated with a vacuum pump and saturated with injected water. The permeability of the core to water was calculated by measuring the pressure and the flow rate in accordance with Darcy’s law. After that, the core was placed in an oven at 54 °C and flooded with oil until no extra water was produced. After the oil flooding, the core was aged for 24 h in the oven at 54 °C and then was flooded with injected water until the water cut reached 98 %. Finally, 0.3 pore volume (PV) of surfactant–polymer solution (0.15 % of surfactant and 1500 mg/L of polymer) was injected followed by water injection to reach a water cut of about 98 %. The injection rate was kept at 0.4 ml/min during the experiment. The results are shown in Fig. 5.
As shown in Fig. 5, with the increase of injected pore volumes, the oil recovery increased gradually. Meanwhile, the water ratio rose constantly until it reached 98 % before the surfactant–polymer slug injection. The oil recovery by water flooding was 48.3 %. After the injection of surfactant–polymer slug of 0.3 PV, the pressure increased rapidly, this is because the slug entered the higher permeability zone, which caused the increase of water flow resistance. The pressure subsequently decreased with the increased oil recovery. The incremental oil recovery was 17.8 % above the water flood, making the total oil recovery reach 66.1 %.
Industrial significance
It is already showed that amino surfactants could be used as oil displacement agents, but different crude oil needed different surfactant formations. The researchers could use other type of surfactants to mix with the synthesized amino surfactant to meet the demand. In the same time, the researchers could try using other amino acids to prepare some novel surfactants.
Conclusions
Diacyl amino acid sodium was prepared and its structure was confirmed. The single use of the surfactant cannot reduce the interfacial tension to ultralow. When combined with sulfonate, the mixed binary surfactant can lower the interfacial tension to ultralow in the presence of polymer. Core flood test showed that the surfactant–polymer can enhance the oil recovery by 17.8 % over the water flood.
References
Qiao WH, Zheng ZB, Peng H, Lu S (2012) Synthesis of and properties of three series amino acid surfactants. Tenside Surf Deterg 49:161–166
Soo EL, Salleh AB, Basri M, Rahman RNEA, Kamaruddin K (2004) Response surface methodological study on lipase-catalyzed synthesis of amino acid surfactants. Process Biochem 39:1511–1518
XU BC, Li JJ (2006) N-Acyl amino acid surfactants. In: Proceedings of the ninth international conference on surfactant and detergent, Shanghai
Infante MR, Perez L, Pinazo A, Clapes P et al (2004) Amino acid-based surfactants. C R Chimi 7:583–592
Xia JD, Xia YM, Nnanna IA (1995) Structure-function relationship of acyl amino acid surfactants: surface activity and antimicrobial properties. J Agric Food Chem 43:867–871
Hou JR, Lui ZC, Zhang SF, Yue XA, Yang JZ (2005) The role of viscoelasticity of alkali/surfactant/polymer solutions in enhanced oil recovery. J Pet Sci Eng 47:219–235
Jing JL, Tang S, Yu T, Gai YJ (2014) Effect of HPAM in ASP flooding wastewater on scaling. Desalin Water Treat 52:6930–6935
Guo WK, Yang ZY, Wu XL, Zhang GY, Wang HF (2006) New type weak alkali surfactant applied to tertiary oil recovery. ACTA Pet Sin 5:75–78
Rosen MJ, Wang HZ, Shen PP, Zhu YY (2005) Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 21:3749–3756
Chan KS, Shan DO (1980) The molecular mechanism for achieving ultra-low interfacial tension minimum in a petroleum sulfonate/oil brine/system. J Disper Sci Technol 1:55–95
Liu DX, Huang HD, Wu YH, Wang YF (1997) Effects of surface activity of nonionic surfactants on POS and its combined system. J Jianghan Pet Inst 14:51–54
Huang HD, Chen YM, Chen Y, He J, Zhang Q (2009) Interfacial activities of petroleum carboxylate and alkylbenzene sulfonate combined system with and without weak alkaline. Oilfield Chem 26:187–190
Wang DM, Cheng JC, Qing YY, Gong WC, Li Q, Chen FM (2000) Viscous-elastic polymer can increase microscale displacement efficiency in cores. SPE Annual Technical Conference and Exhibition, Dallas
Biggs S, Selb J, Candau F (1992) Effect of surfactant on the solution properties of hydrophobically modified polyacrylamide. Langmuir 8:838–847
Acknowledgment
This study was funded by key technical research and application program of increasing and stable 8 million tons of oil production in Huabei oil field (No. 2014E-35-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ren, H., Shi, C., Song, S. et al. Synthesis of diacyl amino acid surfactant and evaluation of its potential for surfactant–polymer flooding. Appl Petrochem Res 6, 59–63 (2016). https://doi.org/10.1007/s13203-014-0094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-014-0094-6