Abstract
Aiming at the characteristics and current status of refineries’ odor pollution, the sulfur containing odor gas–hydrogen sulfide was chosen as a typical object and treated by using the adsorption-catalytic oxidation method in the laboratory. This research focused on the preparation of adsorptive catalyst with high sulfur capacity at room temperature. The results showed that the sulfur capacity of walnut shell activated carbon (WSAC) was up to 351 mg/g, specific surface area was 2,030 m2/g, and the pore volume of microspores was 0.608 mL/g. By comparison with the commercial activated carbon, it is found that the specific surface area, the sulfur capacity, and iodine value of WSAC have been improved significantly. The sulfur capacity of WSAC modified by KIO3 with mass fraction of 1 % was up to 459 mg/g.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, with the industry development, especially the growing scale of petrochemical enterprise development, the odor emissions increase dramatically. The odor damage is very serious and has aroused the widespread interest. Because of the characteristics of the crude oil composition, the oil refining process produces a large amount of the sulfur containing odor substances. The sulfur containing odor substances can cause serious environment pollution. In addition, with the increase of domestic crude oil quality deterioration and sulfur content and the imports of high-sulfur crude oil, the sulfur containing odor pollution becomes worse and has seriously affected the petrochemical enterprise’s survival and development [1]. Therefore, the study on the sulfur containing odor treatment is imperative.
At present, the treatment methods of sulfur containing odor pollution mainly include physical method, chemical method, and biological method [2–5]. The above treatment methods can be used alone, but each of them has its advantages and disadvantages. Comparing with other methods, the adsorption method has the advantage of good effect and simple operation. But, it is mainly restricted by adsorbent adsorption performance and regeneration performance.
More than half of odorous pollutants are sulfur containing gas [6]. In these sulfur containing gas, hydrogen sulfide is the main odor gas. H2S can be detected by most people as low as 0.0047 mg/m3 [7]. From the point of a purification system engineering design, the odor threshold of H2S is 0.18 mg/m3 [8]. Hydrogen sulfide not only can cause a certain degree of corrosion to equipment and line, but also has the serious threat to personal safety. It is also one of the atmospheric pollutants urgently needed to be treated [9]. So, different types of desulfurization catalyst get more attention to eliminate H2S pollution. Previous studies showed that the activated alumina was mostly chosen as the carrier. The metal oxide or a small amount of precious metals could be loaded on the activated alumina to make the supported catalysts [10–12]. Activated carbon has large surface area, huge pore volume, and complex pore structure. So, it can fulfill a dual role as a catalyst for the direct oxidation of hydrogen sulfide to sulfur and as an adsorbent for removing sulfur and its oxides from the gas stream. The selectivity of activated carbon for oxidation of H2S to sulfur is dependent on process conditions, textural characteristics, structural characteristics, and surface chemical characteristics of the carbon catalysts [13, 14].
Considering the cost and treatment effect, this article used the walnut shell waste as raw material and adopted ZnCl2 activation method to prepare the high-specific surface area activated carbon. The preparation conditions of activated carbon were investigated and optimized. The walnut shell activated carbon (WSAC) was also modified and characterized.
Materials and methods
Experimental facility
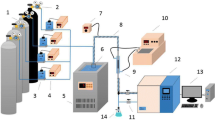
The experimental apparatus included gas generator, gas distribution device, and adsorption device. In the gas generator, sodium sulfide solution and sulfuric acid were used to prepare the hydrogen sulfide gas. The gas generating speed was controlled by adjusting the drip speed of tow solutions.
Hydrogen sulfide gas was mixed with air in the secondary distribution device and then entered into the adsorption device. The adsorption device was controlled by the constant-temperature water bath. The sulfur capacity of activated carbon was calculated by measuring the concentration of import and export hydrogen sulfide gas and the adsorption time. The experimental device is shown in Fig. 1.
Preparation of WSAC
The walnut shells were crushed, removed impurities by a magnet, washed, dried, and sized. The prepared walnut shells were stored in the jar. The ZnCl2 solution was prepared in a certain mass fraction. 300 g prepared walnut shells were added into the ZnCl2 solution, stirred, and allowed to separate for 24 h. After drying, the sample was put into the tube furnace and carbonized at 300 ºC under nitrogen atmosphere. The prepared carbonization sample was steeped in the ZnCl2 solution for 20 min. After extracting and drying, the carbonization sample was put into the muffle furnace. And then, the sample was cooled and boiled with hydrochloric acid for 20 min. The product was washed with deionized water to neutral and put into the oven at 120 ºC.
Desulfurization performance of WSAC
The 10 g WSAC was put into the glass adsorption tube (25 mm in diameter, 330 mm in height), and placed in the 25 ºC constant-temperature water bath. The certain concentration mixed gas was configured by the distribution device. The gas samples were sampled in the intake point and outlet point at regular intervals. When the ratio of gas concentration in the intake point and outlet point was 50 %, the adsorption tube was taken and placed into the drying oven at 98–102 ºC for 2 h. The sulfur capacity of WSAC was calculated by the gaining weight. The hydrogen sulfide concentration was 1,400–1,600 mg/m3, the inlet flow was 350 mL/min and the air speed was 500 h−1.
Performance characterization of WSAC
BET, iodine adsorption value, pH and XPS analysis were used to characterize the WSAC. The iodine adsorption value was measured by the method defined in GB/T 12496.8-1999 [15].
Modification of WSAC
Modification of activated carbon can effectively increase the sulfur capacity. So, the equivolume immersion method was used for WSAC modification at different KIO3 concentrations. The desulfurization capacity of WSAC was also investigated.
Results and discussion
Effect of preparation conditions on desulfurization performance of WSAC
Effect of activator mass fraction on desulfurization performance
In this research, the breakthrough point was defined as the ratio of hydrogen sulphide concentration in the outlet and intake being 10 %, the associated time was the breakthrough time. Figure 2 is the breakthrough curve of WSAC adsorbing hydrogen sulphide. The mass fraction of activator ZnCl2 solution was 20, 30, 40, 50, and 60 %, respectively. The ordinate represented the ratio of hydrogen sulfide concentration in outlet point and the intake point.
Figure 2 shows that with the increase of ZnCl2 mass fraction, the breakthrough time increases. When ZnCl2 mass fraction was 60 %, the breakthrough time was the longest up to 150 min. ZnCl2 had the strong dehydration and could decrease the carbonization temperature [16]. In addition, ZnCl2 could change the decomposition process, inhibited the tar production, and promoted the pores formation. The pores were likely formed when a large quantity of zinc was removed from the hot carbon matrix (boiling point of zinc chloride is 732 ºC [17]). So, with the increasing ZnCl2 mass fraction, the performance of WSAC also increased. Ozdemir et al. also showed that as the impregnation ratio of ZnCl2/grape stalk was increased from 0.5 to 2, the BET surface area of activated carbon prepared increased from 482 to 1,411 m2/g [18].
Effect of activation time on desulfurization performance
Figure 3 shows the breakthrough curve of WSAC adsorbing hydrogen sulphide when the activation time is 30, 60, 80 and 100 min, respectively. The breakthrough time first increased and then decreased with the increase of activation time. When the activation time was 80 min, the breakthrough time was the longest. The volatile content in the walnut shell was high. The volatile content must be removed by carbonizing during the preparation of activated carbon. When the activation time was shorter than 80 min, the inadequate carbonization was not conducive to the pore formation. While the activation time was longer than 80 min, the excessive carbonization resulted in the carbon serious loss and pores distortion. So, the short and long activation times are all disadvantages for WSAC adsorbing hydrogen sulphide.
Effect of activation temperature on desulfurization performance
The activation temperature is a very important parameter which affects the physical characteristics of activated carbon. Figure 4 shows the breakthrough curve of WSAC adsorbing hydrogen sulphide when the activation temperature is 350, 450, 550, and 650 ºC, respectively. The breakthrough time first increased and then decreased with the increase of activation temperature. When the activation temperature was 450 ºC, the breakthrough time was the longest, larger than 150 min. The activation temperature was lower than 700 ºC in Ozdemir et al. [18] research. During the activation process, the action of activator appears in two aspects. On the one hand, activator promotes the pyrolytic reaction and is conducive to the formation of initial pores. On the other hand, the activator is full of pores and it avoids the tar formation. The activated carbon with developed pore structure can be obtained by washing the activator.
Preparation conditions optimization of WSAC
Table 1 lists the preparation factors of WSAC.
In order to optimize the preparation conditions of WSAC, the orthogonal array was designed with activator mass fraction, activation time and activation temperature as factors, the sulfur capacity was the index, as shown in Table 2.
Table 2 shows that the sulfur capacity in all test changes considerably. In test 4, the sulfur capacity was the largest and it was 351 mg/g. The optimal preparation conditions of WSAC were as follows: the activator mass fraction being 40 %, activator time being 60 min, and activation temperature being 450 ºC. Under this condition, the breakthrough time was 183 min and the sulfur capacity was 351 mg/g.
It can be seen that the range of activator mass fraction was the largest (142), while the ranges of activation time and activation temperature are on the same level, which indicated that the activator mass fraction had great influence on sulfur capacity. ZnCl2,, as an activating agent, is very efficient to produce activated carbon with high porosity and high surface area and that the mass fraction has a significant influence on porosity development. According to the pore evolution theory, non-carbonic elements such as oxygen and hydrogen during decomposition change to volatiles and a pressure develops inside the particle due to volatiles accumulation. When these trapped volatiles diffuse out through the particle, the pores are evolved [19].
Modification of WSAC
The impregnation of carbon with transition metals was the well-known way to increase their capacity toward toxic gases [20]. Figure 5 is adsorption desulfurization breakthrough curve of WSAC before and after modification. Comparing with the WSAC before modification, the breakthrough times were all extended after modification. When modifier KIO3 mass concentration was 1 %, the breakthrough time was the longest, longer than 400 min. When modifier KIO3 mass concentration was 5 %, the breakthrough time was only about 300 min. When modifier KIO3 mass concentration was 0.5 %, the breakthrough time was the shortest. This showed that too high and too low modifier KIO3 mass concentration were all disadvantages for modification. If the modifier mass concentration was too low, this led to small active sites on the catalyst surface and low catalytic activation. If the modifier mass concentration was too high, this led to the aggregations of activated centers. In addition, strong oxidization of KIO3 will result in the etching of pore structure and the decrease of catalytic activation.
Figure 6 is the sulfur capacity of WSAC before and after modification at different modifier mass fractions. With 1 % of KIO3, the sulfur capacity of the modified WSAC was the highest, up to 459 mg/g. The sulfur capacities were the same between KIO3 mass fraction of 0.5 and 3 %. Sulfur capacity with the KIO3 mass fraction of 5 % was lower than that of before modification. The reason was that strong oxidization of KIO3 could result in the etching of pore structure and the decrease of catalytic activation.
Performance characterization of WSAC
The BET determination results of WSAC are shown in Table 3 and the physical and chemical properties are shown in Table 4.
Table 3 shows that with the optimum preparation conditions, the specific surface area of WSAC is 2,030 m2/g. The micropore was developed and the average pore size was small, which was very beneficial for adsorption of hydrogen sulfide gas. The pore structure of WSAC was superior to that of activated carbons in Bashkova et al. research [21]. As indicated previously [22], micropores of activated carbon were considered as activated centers for H2S adsorption/oxidation.
From Table 4 it can be seen that, walnut shell active carbon which was prepared under the optimum process conditions, the iodine adsorption value was the largest (more than 1,000 mg/g) and sulfur capacity was 351 mg/g. It was far higher than the sulfur capacity of commercial activated carbon. It was found that the pH after adsorption had a decreasing trend by comparing. The change in the pH after hydrogen sulfide adsorption/oxidation was usually caused by the deposition of H2S oxidation products such as SO2 or H2SO4 [23, 24]. A noticeable increase in surface acidity of commercial activated carbon was usually caused by the presence of sulfuric acid. In fact it was found previously that H2SO4 is the main product of H2S oxidation on virgin carbons and particularly on the wood-based samples [23]. A relatively small decrease in the pH of the WSAC and 1 % KIO3–WSAC in comparison suggested formation of salts and/or elemental sulfur on the surface.
The composition of specific product can be determined by X-ray photoelectron spectroscopy (XPS) characterization methods. The full scan spectra of WSAC after adsorption which was modified by potassium iodate change is shown in Fig. 7.
Figure 7 shows that the strongest peak is the C1s peak, followed by O1s peaks, O KLL peak, S2s peak, and S2p peak. The presence of the peaks explained the main reason for catalyst deactivation after adsorption. The carbon deposits formed on the surface and elemental sulfur product blocked the WSAC pores.
Conclusion
-
1.
The optimal process conditions of WSAC were the activator mass fraction being 40 %, activation time being 80 min, and activation temperature being 450 ºC. Under this condition, the breakthrough time was 183 min and the sulfur capacity was 351 mg/g.
-
2.
1 % of potassium iodate modification was the best, the sulfur capacity can be increased to 459 mg/g, and breakthrough time was up to 400 min.
-
3.
The specific surface area of WSAC was 2,030 m2/g, the average pore size was 1.62 nm, micropore was developed, and it was very beneficial for adsorption of hydrogen sulphide.
-
4.
By modified with 1 % potassium iodate, pH of WSAC was lower. It can be concluded that acidic substances were generated or part of hydrogen sulfide was trapped by physical adsorption.
References
Qu M (2008) Odor pollution sources and treatment technology of domestic oil refineries. Petrochem Saf Environ Prot Technol 24(4):47–50
Kawase M, Otaka M (2013) Removal of H2S using molten carbonate at high temperature. Waste Manag 33:2706–2712
Nowicki P, Skibiszewska P, Pietrzak R (2014) Hydrogen sulphide removal on carbonaceous adsorbents prepared from coffee industry waste materials. Chem Eng J 248:208–215
Perraud I, Ayral RM, Cammarano C, Rouessac F, Hulea V, Ayral A (2014) Sulfidation and sulfur capacity of fine ZnO powder derived from thermal oxidation of mechanosynthesized ZnS powder. Chem Eng J 241:360–365
Ramirez M, Fernandez M, Granada C, Le Borgene S, Gomez JM, Cantero D (2011) Biofiltration of reduced sulphur compounds and community analysis of sulphur-oxidizing bacteria. Bioresour Technol 102:4047–4053
Ministry of Environmental Protection of the People’s Republic of China (1994) Odorous pollutant discharge standard (GB 14554-1993)
Kroschwitz JI, Howe-Grant M (1996) Encyclopedia of chemical technology. Wiley
Cheremisinoff PN (1993) Air pollution control and design for industry. Marcel Dekker Inc, New York
Xiao YH, Wang SD, WU DY, Yuan Q (2008) Catalytic oxidation of hydrogen sulfide over unmodified and impregnated activated carbon. Sep Purif Technol 59:326–332
Musialik-piotrowska A, Mendyka B (2004) Catalytic oxidation of chlorinated hydrocarbons in two-component mixtures with selected VOCs. Catal Today 90:139–144
Alvarez-Galvan MC, de la Pena O’Shea VA, Fierro JLG, Arias PL (2003) Alumina supported manganese- and manganese-palladium oxide catalysts for VOCs combustion. Catal Commun 4(5):223–228
van den Brikn RW, Louw R, Mulder P (1998) Formation of polychlorinated benzenes during the catalytic combustion of chlorobenzene using a Pt/γ-Al2O3 catalyst. Appl Catal B 16(3):219–226
Fang HB, Zhao JT, Fang YT, Huang JJ, Wang Y (2013) Selective oxidation of hydrogen sulfide to sulfur over activated carbon-supported metal oxides. Fuel 108:143–148
Chen J, Xie ZM (2013) Removal of H2S in a novel dielectric barrier discharge reactor with photocatalytic electrode and activated carbon fiber. J Hazard Mater 261(15):38–43
(1999) China State Bureau of Quality and Technical Supervision, test methods of wooden activated carbon-determination of iodine number, GB/T 12496.8–1999
Tsai WT, Chang CY, Lee SL (1998) A low cost adsorbent from agricultural waste corn cob by zinc chloride activation. Bioresour Technol 64(3):211–217
Weast RC, Melvin JA (1981) Handbook of chemistry and physics. CRC Press, Boca Raton
Ozdemir I, Sahin M, Orhan R, Erdema M (2014) Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process Technol 125:200–206
Raveendran K, Ganesh A (1997) Adsorption characteristics and pore development of biomass-pyrolysis char. Fuel 77:769–781
Alder JF, Fielden PR, Smith SJ (1998) The adsorption of hydrogen cyanide by impregnated activated carbon cloth. Part II. Reactivity of impregnated metal carboxylates towards hydrogen cyanide. Carbon 26(5):713–721
Bashkova S, Baker FS, Wu XX, Armstrong TR, Schwartz V (2007) Activated carbon catalyst for selective oxidation of hydrogen sulphide: on the influence of pore structure, surface characteristics, and catalytically-active nitrogen. Carbon 45:1354–1363
Bandosz TJ (2002) On the adsorption/oxidation of hydrogen sulfide on unmodified activated carbon at temperatures near ambient. J Colloid Interface Sci 246(1):1–20
Adib F, Bagreev A, Bandosz TJ (2000) Analysis of the relationship between H2S removal capacity and surface properties of unmodified activated carbons. Environ Sci Technol 34(4):686–692
Adib F, Bagreev A, Bandosz TJ (1999) Effect of pH and surface chemistry on the mechanism of H2S removal by activated carbons. J Colloid Interface Sci 216(2):360–369
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Liu, F., Lu, J. & Zhao, C. Study on the catalysts of sulfur-odor gas pollution treatment. Appl Petrochem Res 4, 337–342 (2014). https://doi.org/10.1007/s13203-014-0069-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-014-0069-7