Abstract
Acrylamide polymer, as one type of fluid loss agent (FLA), had attracted a strong interest in the applied petroleum research because of its outstanding characteristics of thickening and filtration control. The aim of this study was to synthesize a series of binary FLA using different acrylamide monomers and analyze the effect of monomer structure on the fluid loss control performance of FLA. Three kinds of FLA with the monomer of 2-acrylamido-2-methyl propane sulfonic acid (AMPS) were prepared by polymerization of AMPS with acrylamide (AM), N,N-dimethyl acrylamide (DMAM), N,N-diethyl acrylamide (DEAM), respectively, and characterized by FTIR, 1H-NMR and 13C-NMR. The results showed that the FLA prepared by AMPS and DMAM (coded as ADM) showed the best water-retaining capacity, which can be attributed to its moderate rigidity group of −CH3. It was also revealed that the tolerance of cement slurry containing ADM to Ca2+ was stronger than that to Mg2+ and Na+.
Similar content being viewed by others
Introduction
In the field of oil drilling, cement slurry is usually used to reinforce the well wall to provide a stable and safe wellbore (Kelessidis et al. 2009; Kosynkin et al. 2012). To meet the requirements of cementing job, additives such as fluid loss agent (FLA) are used to improve performance of the cement slurry (Dugonjić-Bilić et al. 2011). FLA can control the loss of water from the cement slurry to porous formations and thus prevent the cement slurry from dehydrating. During the past few decades, various FLA including inorganic granular materials, modified natural polymers and synthetic polymers have been developed (Amani et al. 2012). Among them, synthetic polymers were widely studied due to satisfactory performance under complicated formation environment.
2-Acrylamido-2-methyl-propane sulfonic acid (AMPS) with a vinyl group is commonly used as monomer of synthetic FLA due to good thermal stability and low sensitivity to cations (Plank et al. 2006, 2010; Ma et al. 2014). Related researchers developed a series of FLA by copolymerization of AMPS with other monomers to control fluid loss of cement slurry (Peng et al. 2010). It was reported that the copolymer consisting of AMPS and acrylamide (AM) (coded as AAM) had a good performance reducing the fluid loss in oil well cementing (Tao et al. 2011). To further improve the performance of FLA, N,N-dimethyl acrylamide (DMAM) was used instead of AM to copolymerize with AMPS, and the copolymer synthesized (coded as ADM) was found to have a stronger temperature tolerance, resulting from reinforcement of molecular rigidity due to the existence of −CH3 (Guo et al. 2012). However, a more detailed study about performance of ADM was still needed under complicated formation environment. N,N-Diethyl acrylamide (DEAM) as homolog of DMAM has longer carbochain and thus, stronger rigid structure, which gives rise to a supposition that the copolymer consisting of DEAM and AMPS (coded as ADE) could possess better fluid loss control performance.
In this study, three kinds of FLA with similar structure, i.e., AAM, ADM and ADE, were synthesized by the method of aqueous solution polymerization and characterized with fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR). Fluid loss control performance of three kinds of FLA was compared and detailed mechanisms were elucidated.
Experimental section
Materials
AMPS, AM, DMAM, DEAM, NaCl, MgCl2, CaCl2, NaHSO3, (NH4)2S2O8 and Ca(OH)2 were of analytical grade and purchased from Sinopharm Chemical Reagent Co, Ltd. Liquid cement retarder additive (Diacel®HTR100) was provided by Chevron Phillips Chemical Company.
Synthesis of bipolymer FLA
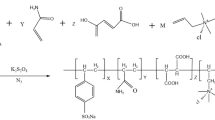
The FLA consisting of AMPS and AM was prepared by redox free-radical polymerization in a four-necked flask. 35 g of AMPS was dissolved in 300 ml of saturated solution of Ca(OH)2 with stirring at 25 ± 2 °C. Ca2+ was introduced in the polymer to increase the molecular weight of FLA through crosslinking role. After all AMPS was dissolved completely, saturated solution of Ca(OH)2 was further added into the solution slowly until pH of the solution reached to 10–10.5. Then, 12 g of AM was put into the solution and stirred for 10 min under N2 atmosphere at 60 °C. The reductant (NaHSO3, 0.05 g/ml, 0.9 ml) was dropped into the solution and stirred for 2 min, and then the oxidant [(NH4)2S2O8, 0.1 g/ml, 1.6 ml] was added slowly and stirred for 20 min. The addition of reductant and oxidant was repeated successively until viscous product was obtained. After the reaction was continued for 1 h, the product was washed with 300 ml of ethanol three times, separated by filter and dried under vacuum at 60 °C for 24 h. The resulting product (i.e., AAM) was ground into fine powder and stored for use. ADM and ADE were synthesized using a similar method, respectively. The synthesis scheme of FLA is shown in Fig. 1.
Characterization of FLA
FTIR (Nicolet AVATAR380, USA) was employed to analyze the surface functional groups of FLA. KBr powder was used for sample preparation. 1H-NMR and 13C-NMR spectra of FLA were obtained by NMR instrument (Bruker Avance III 400 MHz, Germany), and D2O was used as solvent. TGA analysis with a 10 K/min heating rate under nitrogen (Linseis STA PT1000, Germany) was conducted to determine thermal degradation process of FLA.
Cement slurry preparation and fluid loss performance analyses
Cement slurry was prepared according to the API Specification using API Class G, for oil well cement and deionized water. First, FLA was dissolved in deionized water or saline water with different electrolytes (e.g., NaCl, MgCl2, CaCl2), and then cement was added within 15 s. A blade-type laboratory blender manufactured by Waring Products Inc. was used to homogenize the mixture for 35 s at 12,000 rpm. Water-to-cement (w/c) ratio is 0.52. Admixture dosages were stated in % by weight of cement (bwoc). To ensure homogeneous consistency, cement slurry was stirred in an atmospheric consistometer at 27 °C for 20 min.
Static fluid loss of cement slurry was determined following API RP 10B procedure and measured with a high temperature and high pressure (HTHP) filtration device (Fann HTD7169, USA). The fluid volume collected within 30 min was doubled as described by API RP 10B and regarded as API fluid loss of the corresponding cement slurry. Rheological properties of the cement slurry were evaluated by the viscometer (Fann 35SA, USA).
Thickening time of cement slurry was measured under atmospheric pressure using a consistometer (Fann model 275, USA). The cement slurry was poured into the HTHP consistometer cell and the time to reach 70 Bc (Bearden unit of consistency) was sufficient to make the cement slurry unpumpable and was designated as thickening time of the cement slurry. All the experiments were run in triplicate.
Results and discussion
Characterization of FLA
FTIR spectrum of AAM is shown in Fig. 2. The absorption peak at 3,421 cm−1 was attributed to the stretching vibration of N–H in AAM, which was echoed by bending vibration peak of N–H occurring at 1,551 cm−1. The peaks at 2,979, 2,937 and 626 cm−1 were assigned to the stretching vibration of −CH3, −CH2 and C–S of AAM (Guo et al. 2013; Yan et al. 2013), respectively. The stretching vibration peak of C=O in AAM was shown at 1,651 cm−1. Moreover, the peaks at 1185 cm−1 and 1,040 cm−1 should belong to −SO3H group of AAM (Zou et al. 2012; Varaprasad et al. 2010). Comparing with the FTIR spectrum of the monomer AMPS, the characteristic absorption peaks of C=C at 1620 and 944 cm−1 disappeared in the FTIR spectrum of AAM, which demonstrated that C=C bonds of AMPS and AM were opened and the target product AAM was formed. Similarly, the FTIR spectra of ADM and ADE in Fig. 2 also showed that the target product was synthesized by the expected monomers.
1H-NMR (D2O) and 13C-NMR (D2O) spectra of three kinds of FLA are shown in Fig. 3. Detailed spectral analyses based on Fig. 3 are summarized in Table 1. According to Fig. 3a, 1H-NMR peaks of olefinic bond in AMPS and AM, which should occur around 5.7–6.6 ppm, were not found, suggesting that polymerization had been carried out successfully. Similarly, 1H-NMR peaks (around 5.7–6.6 ppm) of olefinic bond in DMAM and DEAM also disappeared, which indicated that DMAM and DEAM successfully polymerized with AMPS, respectively.
Fluid loss control performance of FLA
The performance of various FLA reported in literatures was evaluated at respective experimental conditions, and the lack of comparability causes a need for assessing the performance of various FLA at same conditions. In this study, three kinds of FLA with similar structure, i.e., AAM, ADM and ADE, were selected and fluid loss control performance of FLA was compared under 52 °C. The results are listed in Table 2.
As indicated in Table 2, the shear stress in cement slurry increased with increase in the shear rate. ADM had the best fluid loss control performance among the three kinds of FLA. According to the static filtration equation:
where V t is the filtrate volume, A is filtration area, K is filter cake permeability, ∆p is static pressure difference, R is reduced filter cake volume, t is filtration time and μ is the dynamic viscosity (Plank et al. 2006; Clark 2010).
It could be seen that filtrate volume V t , i.e., the fluid loss, is inversely proportional to viscosity μ 1/2 of the cement slurry. Viscosity μ, is reflected by shear stress as shown in Table 2 (Plank et al. 2010), which is related to the molecular weight and structure of FLA. As mentioned previously, AAM was prepared by polymerization of AM and AMPS. AM has NH2 group with two active hydrogen atoms, which was prone to be initiated by the initiator and produce more copolymer active centers, possibly leading to excessively vigorous reaction and thus lower degree of polymerization (Ma et al. 2014). In contrast, DMAM containing two methyl groups could produce relatively few active centers during polymerization, resulting in higher degree of polymerization and viscosity of ADM. Besides, two methyl groups of DMAM enhanced the hydrophobic association ability of ADM, which improved the blockage effect of cement slurry and thus, decreased fluid loss (Plank et al. 2006; Guo et al. 2012). For ADE, ethyl groups of DEAM could increase steric hindrance, resulting in decrease of degree of polymerization and viscosity of ADE.
Effects of temperature, dosage and salt on ADM performance
Figure 4 shows the effects of temperature on shear stress and fluid loss of cement slurry containing an ADM dosage of 0.8 % bwoc. As observed, ADM on the whole had an excellent fluid loss control performance. With increase in temperature, the fluid loss of cement slurry increased, resulting from the decrease of viscosity of cement slurry as indicated due to change in shear stress. Fluid loss of cement slurry was about 42 ml at 77 °C, indicating a stronger temperature tolerance of ADM and resulting from stronger rigidity of ADM molecule due to the existence of −CH3.TGA spectrum of ADM echoed the results shown in Fig. 4. As observed in Fig. 5, ADM only had a little mass loss before 319 °C, resulting from the volatilization of free water. When the temperature was higher than 319 °C, apparent mass loss began to occur, resulting from the degradation of ADM backbone. The fastest mass loss temperature was 336 °C, confirming a high heat-resistance ability of ADM.
Figure 6 shows the effect of ADM dosage on shear stress and fluid loss of cement slurry. As observed, the fluid loss decreased with the increase of ADM dosage, resulting from the increase of shear stress in cement slurry. However, too high FLA dosage in cement slurry would cause the increase of cementing cost. Selecting an appropriate FLA dosage which was satisfactory in controlling fluid loss was required. In this study, dosage of 0.8 % bwoc ADM was selected in view of the reason that change of fluid loss tends to be mild when dosage was more than 0.8 %. As observed in Fig. 6, the thickening time of cement slurry can be controlled to about 150 min when dosages of ADM were 0.2–1.2 %, showing a stable thickening property of cement slurry which is necessary for keeping the pumping ability of cement slurry.
The salt resistance of cement slurry containing an ADM dosage of 0.8 % bwoc was evaluated in the presence of three kinds of salts including NaCl, MgCl2 and CaCl2 and the results are summarized in Fig. 7. As observed in Fig. 7a, fluid loss of cement slurry increased with increase of NaCl concentration, indicating the negative effects of NaCl on the fluid loss. In salt solution, the charged groups in biopolymer FLA were neutralized by the ions, which reduced the intramolecular charge repulsive force and resulted in the crispation of molecular chain (Zou et al. 2012), viscosity reduction and fluid loss. Fluid loss reached to 146.5 ml when NaCl concentration was 3 %, which is difficult to meet the requirements of cementing job. FLA dosage was raised in the presence of 3 % NaCl to decrease fluid loss of cement slurry, and the result showed that a dosage of 1.0 % bwoc ADM may reduce fluid loss to 48 ml which can usually meet the cementing requirements, indicating excellent salt tolerance of ADM-containing cement slurry. Effects of MgCl2 on fluid loss of cement slurry showed a similar tendency to that of NaCl. Whereas, fluid loss of cement slurry was hardly affected by Ca2+ when Ca2+ concentration was below 1.5 %, indicating strong Ca2+-resistance ability of cement slurry and possibly resulting from the combined action of several factors. On the one hand, Ca2+, like Na+ and Mg2+, can neutralize the charges of FLA and negatively affect the fluid loss. One the other hand, Ca2+ has strong crosslink property, which would draw more FLA to the surface of cement particles and thus, enhance the blockage ability of cement slurry. In this study, two aspects of factors could counteract. Thus, Ca2+ with low concentration hardly affected the fluid loss of cement slurry.
Conslusion
In this study, three kinds of FLA were successfully prepared by polymerization of AMPS with AM, DMAM, DEAM, respectively, and characterized by FTIR and NMR. FLA consisting of AMPS and DMAM (coded as ADM) showed the best water-retaining capacity and had a high temperature tolerance, resulting from strong rigidity of ADM molecule due to the existence of −CH3. Salt-tolerance experiments showed that resistance of cement slurry containing ADM to Ca2+ was stronger than that to Mg2+ and Na+.
References
Amani M, Al-Jubouri M, Shadravan A (2012) Comparative study of using oil-based mud versus water-based mud in HPHT fields. Adv Pet Explor Dev 4:18–27
Clark PE (2010) Analysis of fluid loss data II: models for dynamic fluid loss. J Petrol Sci Eng 70:191–197
Dugonjić-Bilić F, Plank J (2011) Polyelectrolyte complexes from polyethylene imine/acetone formaldehyde sulfite polycondensates: a novel reagent for effective fluid loss control of oil well cement slurries. J Appl Polym Sci 121:1262–1275
Guo JT, Lu HC, Liu SQ, Jin JZ, Yu YJ (2012) The novel fluid loss additive HTF-200C for oil field cementing. Pet Explor Devt 39:385–390
Guo JT, Lu HC, Liu SQ, Jin JZ, Yu YJ, Yu QF (2013) A high temperature retarder HTR-300L applied in long cementing interval. Pet Exp Dev 40:656–660
Kelessidis VC, Papanicolaou C, Foscolos A (2009) Application of Greek lignite as an additive for controlling rheological and filtration properties of water–bentonite suspensions at high temperatures: a review. Int J Coal Geol 77:394–400
Kosynkin DV, Ceriotti G, Wilson KC, Lomeda JR, Scorsone JT, Patel AD, Friedheim JE, Tour JM (2012) Graphene oxide as a high-performance fluid-loss-control additive in water-based drilling fluids. ACS Appl Mater Interface 4:222–227
Ma C, Bu YH, Chen B (2014) Preparation and performance of a lignosulfonate–AMPS–itaconic acid graft copolymer as retarder for modified phosphoaluminate cement. Constr Build Mater 60:25–32
Peng B, Peng SP, Long B, Miao YJ, Guo WY (2010) Properties of high-temperature-resistant drilling fluids incorporating acrylamide/(acrylic acid)/(2-acrylamido-2-methyl-1-propane sulfonic acid) terpolymer and aluminum citrate as filtration control agents. J Vinyl Add Tech 16:84–89
Plank J, Brandl A, Zhai YA, Franke A (2006) Adsorption behavior and effective of poly (N,N-dimethylacrylamide-co-Ca 2-acrylamido-2-methyopropanesulfonate) as cement fluid loss additive in presence of acetone formaldehyde sulfite dispersant. J Appl Polym Sci 102:4341–4347
Plank J, Lummer NR, Dugonjić-Bilić F (2010) Competitive adsorption between an AMPS®-based fluid loss polymer and Welan gum biopolymer in oil well cement. J Appl Polym Sci 116:2913–2919
Tao W, Jie Y, Sun ZS, Wang L, Wang J (2011) Solution and drilling fluid properties of water soluble AM–AA–SSS copolymers by inverse microemulsion. J Pet Sci Eng 78:334–337
Varaprasad K, Ravindra S, Reddy NN, Vimala K, Raju KM (2010) Design and development of temperature sensitive porous poly(NIPAAm-AMPS) hydrogels for drug release of doxorubicin-a cancer chemotherapy drug. J Appl Polym Sci 116:3593–3602
Yan LL, Wang CB, Xu B, Sun JS, Yue W, Yang ZX (2013) Preparation of a novel amphiphilic comb-like terpolymer as viscosifying additive in low-solid drilling fluid. Mater Lett 105:232–235
Zou CJ, Zhao PW, Ge J, Lei Y, Luo PY (2012) β-Cyclodextrin modified anionic and cationic acrylamide polymers for enhancing oil recovery. Carbohydr Polym 87:607–613
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Liu, X., Rao, P., Xiao, W. et al. Synthesis and performance of fluid loss agents based on different acrylamide monomers. J Petrol Explor Prod Technol 5, 409–415 (2015). https://doi.org/10.1007/s13202-015-0154-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-015-0154-1