Abstract
In this study, surface response methodology was employed to investigate the effect of different interacting factors on the removal of fluoride from synthetic water using aluminum electrocoagulation (Al-EC) and iron electrocoagulation (Fe-EC) in different reactors. Box–Behnken design of a Design Expert version 11 was used for the optimization and evaluation of the process independent variables: applied electric density, initial pH, initial fluoride concentration and treatment time on the efficiency of fluoride removal as a response. Results showed that the effect of current density and initial fluoride concentration was significant model terms for fluoride reduction in Fe-EC and Al-EC reactors, respectively. The Al-EC reactor model presented the R2 value of 79.2% while Fe-EC presented R2 value of 75.8%, showing that both models can predict the response well. The reduction by 94% (initial concentration of 16 mgF/L) was established at optimal operating parameters of 18.5 mAcm−2, pH 6.80 in 50 min using Al-EC. On the other hand, 16 mgF/L was reduced by 92% to 1.28 mgF/L in Fe-EC reactor at optimal condition of 6.5 mAcm−2, pH 6.50 in 50 min. Experimental results correlated well to the model predicted results that were 95 and 94% for Al-EC and Fe-EC, respectively. Both reactors manage to reduce fluoride to a level recommended by WHO (≤ 1.5 mg/L) for drinking purpose.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
It is approximated that 90% of population of people living in African Rift valley is affected with dental and skeletal fluorosis due to consumption of groundwater water containing elevated levels of fluoride (Fawell et al. 2006). Despite availability of various methods such as adsorption, ion exchange, filtration, coagulation and membrane processes for defluoridation (Mohapatra et al. 2009), in the African Rift Valley zone, people are mainly using bone char and Nalgonda methods (Osterwalder et al. 2014). This is due to their low cost, effectiveness and availability of materials (García-Lara and Montero-Ocampo 2010). However, the methods face rejection by some users because of odor and offensive taste especially when there is poor production of bone char (Fawell et al. 2006). On the other hand, Nalgonda method requires high chemical coagulants for effective fluoride removal, resulting into a high sludge production that becomes a challenge in their disposal. Thus, electrocoagulation has demonstrated to be an alternative treatment method to avoid high production of sludge, odor and smell as on chemical coagulation.
Electrocoagulation (EC) method involves electricity for in situ generation of coagulant that destabilizes chemical pollutants in water (Moussa et al. 2017). The common electrodes include aluminum, titanium, zinc, copper and iron; aluminum and iron being superior than the others, due to their efficiency and availability (Moussa et al. 2017). In EC, the metal ion dissociates from the anode (Eqs. 1 and 3) while water at the surface of the cathode dissociates into H+ and OH− ions, the two react to form metal hydroxide (coagulant) as shown in Eqs. (2) and (4) for aluminum and iron, respectively. The coagulant then provides surface site for charge neutralization, adsorption, coagulation and co-precipitation which remove fluoride by floc formation (Kobya et al. 2006).
The efficiency of EC experiments depends on interaction among several independent factors, namely the current applied, initial pH, treatment time, conductivity of the sample and initial pollutant concentration (Moussa et al. 2017). Recently, optimization of EC operation factors has been performed by varying a single factor while keeping other factors fixed at a specific set of conditions. This process is time consuming and requires high number of runs leading to poor optimization due to ignoring of some interaction (Tir and Moulai-Mostefa 2008). The mentioned shortfalls can be easily solved by a response surface methodology (RSM). The method uses various designs such as factorial design (FD) (Daghrir et al. 2013), central composite design (CCD) (Amani-Ghadim et al. 2013), D-optimal design (DOP) (Tir and Moulai-Mostefa 2008), and Box–Behnken design (BBD) (Behbahani et al. 2011). The first three designs face a challenge of higher number of experiments required and thus become costly. On the other hand, the BBD provides the same information as the others but with the advantage of less number of experiments (Box and Behnken 1960). Furthermore, the BBD does not contain combinations in which all factors are simultaneously at their highest or lowest levels, which is important in avoiding experiments performed under extreme conditions that may cause unsatisfactory results (Box and Behnken 1960), hence selected for this work.
The aim of this study was to optimize and model fluoride removal from aqueous solutions in two separate electrocoagulation reactors, Al-EC and Fe-EC, establish and compare their efficiencies on fluoride removal and to determine relationship between responses and four quantitative variables (initial pH, initial fluoride concentration, current density and treatment time) using Box–Behnken design (BBD).
Materials and methods
Material preparation
The fluoride solution was prepared using sodium fluoride (NaF), where 2.21 g of sodium fluoride was dissolved in 1000 mL of distilled water to make 1000 ppm of fluoride in the stock solution. Then, serial dilution was used for a specific concentration preparation. Meanwhile the electrodes, aluminum and iron electrodes plates of 10 cm × 2 cm, each were pre-cleaned by rubbing with sandpaper, rinsed in NaOH (2 M) and HCl (2 M) to remove any particles attached on the surface before washing with distilled water. The electrodes were then dried at 105 °C before the start of experiments. After each experiment, electrodes were dissolved in 1 M hydrochloric acid for 10 min to remove any remaining particles on the surface that may reduce EC performance.
Design of experiment
In this study, the Box–Behnken design BBD was selected for the optimization of EC process used for the fluoride reduction. The four-factorial and a three-level BBD with five replicas at the center point leading to twenty-nine (29) experiments was employed for response surface modeling in this study. The variables (independent factors) chosen were: the applied electric current density (A), initial pH of the water sample (B), treatment time (C) and initial concentration of fluoride (D). Meanwhile, the percentage fluoride reduction was considered as dependent factor (response).
The electrocoagulation set up and sample analysis
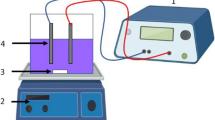
The EC experiments were carried-out in a batch mode reactor (Fig. 1). The reactions were conducted in 800 cm3 beaker, with a working volume of 600 cm3. The two aluminum electrode plates with a total surface area of 80 cm2 each were connected to other two aluminum as cathode in a monopolar parallel connection MP-P. This makes the effective surface area to volume ratio the reactors (S) to be 0.15 cm−1. Similar reactor set up for the iron electrode Fe-EC was employed. The anodes and cathodes were placed alternatively and parallel to each other at a specific distance of 1.5 cm. Thereafter, the end poles of sacrificial electrodes were connected to the anode in a direct current (DC) power source, (BK Precision 1796).
The experiments were then performed in series of combination of different operation parameters as shown in Table 2. On the other hand, the conductivity of water sample was improved by addition of small amount of sodium chloride (200–300 mg) as it has ability to eliminate the passive films on aluminum electrodes according to corrosion pitting phenomenon (Mansouri et al. 2011). After each set of experiment, samples were taken then filtered (Whatman filter paper 0.6 micron) and analyzed using ion selective electrode (ISE) for final fluoride concentration. Percentage of fluoride removal was then calculated (Eq. 5).
where F0 and Ft are initial and final fluoride concentration, respectively.
Results and discussion
The actual values of process variables and their variation limits shown in Table 1 were selected based on the values obtained from various studies as well as in preliminary experiments. Results of the experiments performed are presented in Table 2 showing the actual and predicted reduction of fluoride at different combinations of operating parameters using aluminum and iron electrodes, respectively.
Evaluation of the data
For the evaluation of experimental data on their validity in optimization of operation parameters, the response variable was fitted by a second-order model in the form of quadratic polynomial equation given by Eq. (6) as proposed by (Khedmati et al. 2017).
In this equation Y is the response variables (fluoride reduction) and b0, bi, bii, and bij are constant coefficients of intercept, linear, quadratic and interactive terms, respectively and Xi and Xj represent the four independent variables (current density, initial pH, treatment time, and initial concentration). Experimental data shown in Tables 2 were analyzed using Design-Expert version 11 program including ANOVA and regression to obtain the interaction between the process variables to the response. Two-dimensional, contour plots and three-dimensional curves of the response surfaces were developed using the same program to explain the interactions among variables and their effect to the fluoride reduction.
Optimization procedure
Table 3 shows the key findings after analysis of variance and regression of the data set presented in Table 2 to assess the validity of the model prediction for Al-EC and Fe-EC, respectively. In this study, the coefficients of the model for constant terms, cubic effects, quadratic effects and interaction effects were evaluated. The model low p-values of 0.0087 and 0.022, and large F-values 3.81 and 3.07 imply that at least one of the terms in each model has a significant effect on the response in Al-EC and Fe-EC setups, respectively (Karimifard and Moghaddam 2018). The lack of fit p value of 0.1125 for Al-EC and 0.7344 for Fe-EC both being ≥ 0.05 made lack of fit not significant to both setups (Karimifard and Moghaddam 2018). As seen from Table 3, the initial pH (B), and initial fluoride concentration (D) are both significant to the model (p < 0.05), where initial fluoride concentration being the most significant p < 0.0001 to Al-EC. For the Fe-EC, the current density appears to be the only significant factor p = 0.01. The interaction between initial pH and initial concentration (BD), and between initial concentrations themselves (D2) are highly significant factors (p < 0.05) as compared to other interaction terms in both reactors.
The regression coefficient R2 of 0.79 and 0.75 for Al-EC and Fe-EC, respectively, show that the interaction among the factors in experimental data to both models can fairly predict the response (Karimifard and Moghaddam 2018). The adequate precision which is 7.8 for Al-EC and 6.4 for Fe-EC also signifies that the signal-to-noise ratio is appropriate and adequate. The adjusted R2 of 0.58 and 0.50 both being lower than the R2 values suggest that the new number of factors included in the model in trying to modify it could not improve the model (Karimifard and Moghaddam 2018). The R2 values observed indicate that the regression models explained electro-defluoridation fairly. Hence, the response surface model developed in this study for predicting fluoride removal efficiency was considered to be satisfactory.
From the normal probability plot of residuals as shown in Fig. 2a and b it can be ascertained with assumption that both models were relatively satisfactory as the points in the plot form fairly straight-line. In the case of actual residual against fit plot, for a model to be reliable, no series of increasing or decreasing points patterns such as increasing residuals with increasing fits and a predominance of positive or negative residuals should be found. Furthermore, the data shown in Table 3 can also be observed and confirmed in Fig. 3a and b showing alignment between the residuals and predicted fluoride reduction. The predicted and actual plots Fig. 4a and b further indicate the close agreement between the actual and predicted results, suggesting that the model could well predict within the range of operation parameter. Therefore, it can be concluded that the quadratic model of the response surface developed in this study correlating fluoride reduction with process variables is best suited to explain the experimental data of electrocoagulation process.
Effect of individual operating parameters
The effect of individual operating parameters, current density, initial pH, treatment time and initial fluoride concentration considered in this study is shown in the plots presented in Figs. 5 and 6 for Al-E and Fe-EC, respectively. The parameters were measured as the function of fluoride reduction. These parameters are known to be key factors for operation of electrocoagulation system (Moussa et al. 2017).
The effect of current density
From the plots, it can be observed that, from the lower current density there is a linear increase on fluoride reduction to a maximum current of 38.5 and 16 mA/cm2 for Al-EC and Fe-EC, respectively. At these points, there is maximum removal of fluoride by 97% for Al-EC and 95% for Fe-EC. According to Faraday’s law (Eq. 7), as the applied current increases, dissolution of aluminum and iron ions also tend to increase, leading to high formation of coagulant Al(OH)3 and Fe(OH)3, bubble and floc size (Mehmet Kobya et al. 2011).
where W is the theoretical amount of aluminum or iron produced by current I (A) passed for a period of time t (s), M is molecular mass (Al; 26.98 g/mol: Fe; 55.84 g/mol), N is number of electrons transferred (N − 3 for Al; 2 for Fe), F is Faradays constant (96,485 C/mol).
However, removal efficiencies started to decline when the J ≥ 16.1 mAcm−2 for Fe-EC and ≥ 50 mA/cm2 for Al-EC. Higher current density means high generation of coagulant near the electrode that restrict the free movement of particles causing overpotential to build up in the reactor due to ohmic drop (Nasrullah et al. 2012). This prevents further adsorption of the fluoride ion as observed at J ≥ 16.1 mAcm−2 and J ≥ 50 mAcm−2. The ohmic drop was also confirmed that at the same treatment time both reactors attained the same amount of coagulant dosage of 0.000063 g/L. It can be ascertained that at the coagulant dose ≥ 0.000063 g/L no further fluoride reduction can be achieved as the kinetic of particles become restricted. The lower ohmic drop in Fe-EC than Al-EC is attributed to the higher molar mass of iron that achieved the same amount of coagulant as aluminum at lower electric current by a factor of ~ 0.3.
Initial fluoride concentration
The influence of initial fluoride concentration appears to be similar to both Al-EC and Fe-EC systems. For Al-EC, at a lower fluoride concentration starting from 2 to 26 mgF/L, there is increase in fluoride reduction rate from 78 to 95%. At this point, the ratio between adsorbate (F−) and adsorbent Al(OH)3 and Fe(OH)3 is very low and permits rapid uptake of fluoride. But as the initial fluoride level reaches ~ 27 mgF/L, the removal rate slows down and remains constant as its limited by availability of free surfaces on the coagulant for further uptake (Vasudevan et al. 2009). However, above 30 mgF/L, the reduction appears to decrease to 92%. Likewise, for Fe-EC, the removal increases with initial fluoride concentration from 2 mgF/L and reaches maximum at 21 mgF/L. Above this level, there is decrease in removal efficiency to 91 from 95% at highest initial concentration of 40 mgF/L. The decrease in removal is due to competition for complexation sites which limit fluoride removal and subsequent further stirring tends to lead to desorption (Vasudevan et al. 2009). This study also found that Fe(OH)3 reaches saturation point at a lower fluoride concentration of 21 mgF/L compared to 27 mgF/L of Al(OH)3.
Initial pH of the water sample
From the plot, it is interesting to see that the fluoride removal by Al-EC increases 78–96% as the initial pH of the sample increases. The maximum fluoride removal is attained at pH 7.5–8.5. This is because at a lower pH ~ 4, there is a higher hydrogen ion concentration leading to formation of soluble HF. At a pH ≥ 4, amorphous hydroxide Al(OH)3 starts to form due to hydrolysis of water at the cathode, the amount of this amorphous further increases with pH as more hydroxide is added that lead to more uptake of fluoride. The amorphous Al(OH)3 has minimum solubility and is finally polymerized into Aln(OH)3n, which results into dense flocs formation with large surface area (Holt et al. 1999) for high fluoride uptake. However, at pH ≥ 9, the removal remains constant signifying formation of negatively charged aluminum hydroxide Al(OH) −4 that repels with the fluoride ion in the solution (Mechelhoff et al. 2013). Meanwhile, Fe-EC behaves differently, and the highest reduction is attained at pH 4.5–5.5 beyond which there is insignificantly slow down from 95 to 92% at pH 9. In acidic medium, there is higher dissolution of iron (Cañizares et al. 2007) leading to the formation of high amount coagulant Fe(OH)2. Study by (Sasson et al. 2009) also revealed significant high Fe2+ dissolution rate at pH values of 5–6 than that at pH 8–9. In this context, it is important to note that the chemical dissolution of the electrode surfaces is promoted at alkaline pHs (for the case of aluminum) and at acidic pHs (for the case of iron).
Treatment time
In this investigation, the reduction efficiencies in both aluminum and iron electrodes appeared to exhibit exponential relation to time. For Al-EC, there is an increase in fluoride reduction from 90% at 10 min to 93% with the maximum removal at 40 min. Above this time, there is significant decrease on removal efficiency to 91.7% in 60 min. For the Fe-EC, more similar trend is observed; however, the removal efficiency remained constant at a longer time of 50–60 min. This indicates that further treatment time beyond 40 min and 50 min for Al-EC and Fe-EC, respectively, has negative influence on the fluoride removal. For both cases during the first 10 min, there is appreciable enough in situ generated hydroxides of aluminum and iron to reduce fluoride by 90%. The longer time needed for iron to reach maximum fluoride reduction may be due to small current density applied during this investigation as compared to aluminum electrode. This is attributed to high adsorption capacity of in situ generated aluminum coagulant for fluoride ion (Smedley et al. 2003).
Combined effect of operational parameters on fluoride removal efficiency
The current density is considered as the key factor controlling efficiency of any electrocoagulation reaction (Moussa et al. 2017). However, in this study other factors when correlated to current density are observed to limit fluoride removal in both reactors’ Figs. 7 and 8a–c. Generally, the maximum fluoride removal observed is ~ 96 and ~ 94% for Al-EC and Fe-EC, respectively. At a lower treatment time ≤ 39 and ≤ 45 min and initial fluoride concentration ≤ 27 and ≤ 21 mgF/L for Al-EC and Fe-EC, respectively, the removal increased at all current density applied. However, above these levels there is significant reduction in fluoride removal, despite the increase in current density. This indicates that the treatment time and initial fluoride concentration are limiting factors in their higher levels. Despite further dissolution of Al3+ and Fe2+ as the function of current and time, when the F−/Al(OH)3 and F−/Fe(OH)2 is ≥ 1 adsorption cannot take place (Vasudevan et al. 2009). Figures 7 and 8d also verify similar finding that an increase in treatment time at initial concentration ≥ 27 and ≥ 21 mgF/L has no significant impact on the fluoride removal. At any suggested operation condition of current density, initial pH and treatment time, the maximum initial fluoride that can be removed effectively (i.e. ≤ 1.5 mgF/L) is 27 and 21 mgF/L for Al-EC and Fe-EC, respectively.
Surface plots with contour as a function of a treatment time and current density at pH 6.5 and initial concentration 21 mg/L, b initial concentration and current density at pH 6.5 and treatment time 35 min, c pH and current density at initial concentration of 21 mgF/L and time 35 min, d concentration and time at current density 28 mAcm−2 and pH 6.5
Surface plots with contour as a function of a treatment time and current density at pH 6.5 and initial concentration 21 mgF/L, b initial concentration and current density at pH 6.5 and treatment time 35 min, c pH and current density at initial concentration of 21 mgF/L and time 35 min, d concentration and time at current density 12.4 mAcm−2 and pH 6.5
Model equations
Final equation model in term of coded factors Al-EC
The final regression model in terms of coded factors has been expressed by the following second-order polynomial equation:
Final equation in terms of actual factors
In terms of actual factors, an empirical relationship between F− removal efficiency and the variables has been expressed by the following second-order polynomial equation:
Fluoride removal by Al-EC and Fe-EC optimization
The main objective of optimization was to determine the optimum values of variables for fluoride removal with EC from the model obtained using experimental data. In both reactors Al-EC and Fe-EC, the operating parameters were chosen so as to maximize fluoride reduction at the minimal current density, pH, initial fluoride concentration and treatment time were left at a range. Several sets of experiments were suggested by the model and two of them were performed.
From the experimental results shown in Table 4, Al-EC achieved the reduction of initial 16–0.92 mgF/L that satisfies WHO minimum fluoride level for drinking water. This 94.25% reduction was achieved at optimal conditions of initial fluoride concentration of 16 mgF/L, current density 18.5 mAcm−2 initial pH 6.8 and treatment time 50 min. For the Fe-EC, the best removal was predicted to be 94% at initial fluoride concentration of 16 mgF/L, current density 6.25 mAcm−2 in 60 min. The observed result at this optimum condition was 92.00% that confirmed to be close to the predicted removal.
Conclusion
In this research work, the effects of four main parameters in the electrocoagulation process including initial pH, initial fluoride concentration, current density and reaction time were evaluated and compared on the reduction of fluoride in Al-EC and Fe-EC reactors. The results showed that the initial fluoride concentration was significant factor on Al-EC reactor and current density was the only main factor in Fe-EC reactor. The ANOVA results presented fairly R2 values of 79.23 and 75.45% for Al-EC and Fe-EC fluoride removal, respectively, indicating the good accuracy of the polynomial models for both models. From the optimization, the Al-EC attained 94% fluoride removal at initial pH of 6.8, initial fluoride concentration of 16 mgF/L, current density of 18.5 mAcm−2 and reaction time of 50 min. The Fe-EC attained 92% removal at pH of 6.5, initial fluoride concentration of 16 mgF/L, current density of 6.25 mAcm−2 and reaction time of 60 min. It can be ascertained that both electrodes Al and Fe attained reduction of fluoride to a permissible WHO standard for drinking water (≤ 1.5 mg/L) at optimized operational parameters.
References
Amani-Ghadim AR, Aber S, Olad A, Ashassi-Sorkhabi H (2013) Optimization of electrocoagulation process for removal of an azo dye using response surface methodology and investigation on the occurrence of destructive side reactions. Chem Eng Process 64:68–78
Behbahani M, Moghaddam MRA, Arami M (2011) Techno-economical evaluation of fluoride removal by electrocoagulation process: optimization through response surface methodology. Desalination 271(1–3):209–218. http://www.sciencedirect.com/science/article/pii/S0011916410009689
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475
Cañizares P et al (2007) Study of the electrocoagulation process using aluminum and iron electrodes. Ind Eng Chem Res 46(19):6189–6195
Daghrir R, Drogui P, Zaviska F (2013) Effectiveness of a hybrid process combining electro-coagulation and electro-oxidation for the treatment of domestic wastewaters using response surface methodology. J Environ Sci Health Part A Toxic Hazard Subst Environ Eng 48:308–318
Fawell J et al (2006) Fluoride in drinking-water. IWA publishing, London
García-Lara AM, Montero-Ocampo C (2010) Improvement of arsenic electro-removal from underground water by lowering the interference of other ions. Water Air Soil Pollut 205(1–4):237
Holt P, Barton G, Mitchell C (1999) Electrocoagulation as a wastewater treatment. Third Annu Aust Environ Eng Res Event 1000:41–46
Karimifard S, Moghaddam MRA (2018) Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review. Sci Total Environ 640:772–797
Khedmati M, Khodaii A, Haghshenas HF (2017) A study on moisture susceptibility of stone matrix warm mix asphalt. Constr Build Mater 144:42–49. https://doi.org/10.1016/j.conbuildmat.2017.03.121
Kobya M et al (2006) Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination 190(1–3):201–211
Kobya M et al (2011) Treatment of potable water containing low concentration of arsenic with electrocoagulation: different connection modes and Fe–Al electrodes. Sep Purif Technol 77(3):283–293
Mansouri K, Ibrik K, Bensalah N, Abdel-Wahab A (2011) Anodic dissolution of pure aluminum during electrocoagulation process: influence of supporting electrolyte, initial pH, and current density. Ind Eng Chem Res 50(23):13362–13372
Mechelhoff M, Kelsall GH, Graham NJD (2013) Super-faradaic charge yields for aluminium dissolution in neutral aqueous solutions. Chem Eng Sci 95:353–359
Mohapatra M et al (2009) Review of fluoride removal from drinking water. J Environ Manag 91(1):67–77
Moussa DT, El-naas MH, Nasser M, Al-marri MJ (2017) A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J Environ Manag 186:24–41. https://doi.org/10.1016/j.jenvman.2016.10.032
Nasrullah M, Singh L, Wahid ZA (2012) Treatment of sewage by electrocoagulation and the effect of high current density. Energy Environ Eng J 1(1):27–31
Osterwalder L, Annette Johnson C, Yang H, Johnston RB (2014) Multi-criteria assessment of community-based fluoride-removal technologies for rural Ethiopia. Sci Total Environ 488:532–538
Sasson MB, Calmano W, Adin A (2009) Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J Hazard Mater 171(1–3):704–709
Smedley PL, Zhang M, Zhang G, Luo Z (2003) Mobilisation of arsenic and other trace elements in fluviolacustrine aquifers of the Huhhot Basin, Inner Mongolia. Appl Geochem 18:1453–1477
Tir M, Moulai-Mostefa N (2008) Optimization of oil removal from oily wastewater by electrocoagulation using response surface method. J Hazard Mater 158(1):107–115
Vasudevan S, Lakshmi J, Sozhan G (2009) Studies on a Mg–Al–Zn alloy as an anode for the removal of fluoride from drinking water in an electrocoagulation process. Clean Soil Air Water 37:372–378
Acknowledgements
The authors are kindly acknowledging the Department of Chemistry (Addis Ababa University) for providing laboratory space and laboratory facilities in achieving this work.
Funding
This work was supported by Africa Centre of Excellence in Water Management (ACEWM) funded by the World Bank ACEII Project for capacity building with Grant Number GSR 9808/10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyangi, M.J., Chebude, Y. & Kilulya, K.F. Fluoride removal efficiencies of Al-EC and Fe-EC reactors: process optimization using Box–Behnken design of the surface response methodology. Appl Water Sci 10, 214 (2020). https://doi.org/10.1007/s13201-020-01297-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01297-x