Abstract
Conforming to the factor characteristics of electricity–water cogeneration power plants, an improved multieffect desalination (MED) and reverse osmosis (RO) are the most current techniques for seawater desalination. A principal change between these operations consists of their various energy requirements, thermal energy for MED and mechanical energy for RO plants. The main improvement ideas of RO and MED are the recovery of large quantities of heat produced during the production of sulfuric acid, which exhibits an exothermic reaction where a part of this heat will be used for the thermal power plant and subsequently reverse osmosis and another amount of heat recovery system will be used to desalinate seawater by the MED system. In this work, the sulfuric acid is used to recover heat energy; this energy is used in a hybrid system of RO–MED in order to desalinate the seawater. A thermodynamic steady-state study of this system is investigated to select the optimum cogeneration system. These improvements could make the benefits of cogeneration to the energy consumption of an RO and MED which will be remarkably reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water and energy are two inseparable products that govern the life of humanity and civilization develops (Mourad et al. 2018). The facility to produce potable water economically is the crucial focus of seawater desalination research. There are numerous methods to desalinate water, including reverse osmosis, multistage flash distillation and multieffect evaporation; seawater desalination is an attractive idea for addressing the water supply shortages. Water evaporation and membrane desalination methods are two major methods; reverse osmosis (RO) is the most well-known example of membrane desalination and requires low energy supply (Saifaoui et al. 2016). Nevertheless, it has a high maintenance cost and produces water-containing remnant bromides and chlorides (Aroussy et al. 2016b). Multieffect desalination (MED), multistage flash (MSF) and multieffect desalination with thermal vapor compression (MED-TVC) are thermal methods that can use nonpotable water with various characteristics such as excessive salinity (Amry et al. 2016).
On the other hand, countries such as those in the sub-Saharan region have to rely on imported oil for electricity generation and face frequent periods of severe draught. Analogous conditions prevail in small- or medium-size islands, which are not connected to national electricity grids (e.g., islands of the Aegean Sea). In many such cases, electricity is produced by diesel generators which convert less than half of the energy content of the fuel into electricity. The remainder energy is evacuated as waste heat by the combustion gases (at approximately 350 °C) and the cooling liquid (at approximately 80 °C) of the diesel engine. In the entire scientific world, great efforts have been made for a rational improvement of design and operation criteria for desalination plants, oriented to all the main aspects of processes that are water, chemical and physical treatments, membrane materials, thermodynamics of heat recovery and rejection and different layouts for hybrid RO–MED plants (Khoshgoftar Manesh et al. 2013). New frontiers for desalination plants will be opened from the expected widespread use of modular components to be produced on a large scale with consequent capital cost abatement.
Among the thermal desalination methods which can use waste heat, multieffect evaporation (MEE) and multieffect evaporation with thermal vapor compression (MEE-TVC) have lower corrosion and scaling rates, lower capital cost, longer operation life and require less pumping power (Lovato et al. 2001). They have therefore been studied frequently and implemented in several increasingly larger installations. Some of these studies as well as a few others of particular interest are presented in ascending chronological order in the following paragraph.
In this circumstance, the sulfuric acid production is an exothermic process (Aroussy et al. 2016a), in which the heat is recovered from absorption process of sulfuric acid by heat recovery system (HRS), with negligible temperature losses and minimum space requirements. The heat recovered is used for district heating of cities, boiler feed water preheating or as heating media in connected processes or adjacent plants. Indeed, it provides the entire need for sulfuric acid and steam for the production of phosphoric acid, fertilizer and the electrical energy required to power the plant. In this work, the sulfuric acid is used to recover heat energy in power plant which is used in a hybrid system of RO–MED in order to desalinate the seawater. A thermodynamic steady-state study of this system is investigated to select the optimum cogeneration system.
Desalination methods
A desalting device essentially separates saline water into two streams: the freshwater stream (low salts) and the concentrate or brine stream (high salts). The device requires energy to operate and can use a number of different technologies for the separation (Fig. 1).
Multieffect distillation
Multieffect distillation uses multiple vessels of decreasing pressure, similar to multistage flash distillation (Fig. 2). The major difference is that evaporation occurs at the heat exchanger surface, which can lead to fouling.
Reverse osmosis
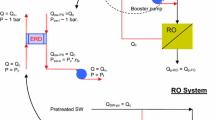
Reverse osmosis is a pressure-driven process, which forces water through a selective membrane, leaving salts behind. The major energy required for desalting is for pressurizing the feed water (Fig. 3).
The share of RO membrane process is rapidly increasing with the time compared to distillation processes as it consumes much less energy and thus less cost (Fig. 4).
Cogeneration system
On the other hand, thermal power generation is not very effective if the waste heat is not recovered from the power plant (de Gunzbourg and Larger 1999; El-Nashar 2001). Thus, for the recovery of these, it increases the overall plant efficiency by getting more useful energy per unit of fuel burned. To this end, it fills more needs rejecting less heat, fewer greenhouse gases and fewer pollutants. During the power generation using combustibles, the wasted heat energy is recovered by a cogeneration system, which is divided in two main energies: mechanical energy and thermal energy as mentioned in Fig. 5.
Numerical results
In this part, we will present the different systems that can be used from heat recovery in production of sulfuric acid as in the diagrams (Fig. 6).
The steam produced by the boiler field runs a Rankine power cycle to produce electricity for the RO system and auxiliary components of the MED system after steam of acid sulfuric production (thermal energy) is used to power the other MED desalination system which shows the energy efficiency of our system. The Thermodynamic models of each of the two main system components (RO and MED) were constructed in Engineering Equation Solver (EES) and solved numerically. The effect of all parametric system is investigated in this study, especially the reverse osmosis system.
RO system model
In the RO process, the solution declined by the membrane, also called brine, is more concentrated than the power solution. Its pressure is slightly smaller than the working pressure, although it is still very important. The same capacity can be collected using a recovery unit to generate mechanical energy to drive pumps.
In this paper, we assume that (Cardona and Piacentino 2004):
Flow rate is steady;
Salt and saltwater are incompressible substances;
Salinity at the inlet is constant (point 5);
Kinetic and potential energies are negligible;
Terms of saltwater at the entrance match ambient conditions (reference conditions);
Performance of all pumps and turbines is set at the same value of 75%;
In the former case (system # 1), the RO subsystem embodying a hydraulic turbine is mechanically coupled with the Rankine cycle subsystem, as shown in Fig. 7.
Thermodynamic study in steady state
System # 1:
Boiler:
The first law of thermodynamics in steady state (Bouzayani et al. 2007; Cangel and Boles 2002) for the volume control of the boiler, not taking into account the heat loss to the outside, is expressed as:
-
The reversible work of the boiler in steady state is given as:
$$w_{\text{r,boiler}} = \frac{{\dot{W}_{\text{r,boiler}} }}{{\dot{M}}} = - T_{0} \cdot \left( {\frac{{q_{1} }}{{T_{\text{s}} }} + (S_{1} - S_{2} )} \right)$$
Steam turbine:
-
The first law of thermodynamics in steady state for superheated steam control volume contained between sections 2 and 3 of the steam turbine, not taking into account heat exchanges with the outside, is expressed as:
$$w_{\text{TV}} = h_{2} - h_{3} = \eta_{\text{TV}} \cdot (h_{2} - h_{3s} )$$ -
The reversible work of the steam turbine in steady state is expressed as:
$$w_{\text{r,TV}} = w_{\text{TV}} - T_{0} \cdot (S_{2} - S_{3} )$$
Condenser:
-
The first law of thermodynamics in steady state for the vapor control volume contained between sections 3 and 4 of the condenser, by not taking into account the heat exchange with the outside, is expressed as:
$$q_{3} = \frac{{\dot{Q}_{3} }}{{\dot{M}}} = h_{4} - h_{3}$$ -
The reversible work of the capacitor in steady state is given as:
$$w_{\text{r,cond}} = - T_{0} \cdot \left( {\frac{{q_{2} }}{{T_{0} }} + (S_{3} - S_{4} )} \right)$$
Pump 1:
-
The first law of thermodynamics steady state for the volume control of pure liquid water contained between sections 1 and 4 of the pump Pm1, not taking into account the heat exchanges with the outside, is expressed as:
$$w_{\text{Pm1}} = \frac{{\dot{W}_{\text{Pm1}} }}{{\dot{M}}} = \frac{{P_{1} - P_{4} }}{{\rho_{\text{W}} \cdot \eta_{\text{Pm1}} }} = h_{1} - h_{4}$$ -
Entropy in point 4 is expressed as:
$$h_{4} = h_{1} + \frac{{P_{4} - P_{1} }}{{\rho_{\text{W}} \cdot \eta_{\text{Pm1}} }}$$ -
The reversible work of pump Pm1 in steady state is given as:
$$w_{\text{r,Pm1}} = h_{4} - h_{1} - T_{0} \cdot (S_{4} - S_{1} )$$
Pump 2:
-
The first law of thermodynamics in steady state for seawater control volume contained between sections 5 and 6 of the pump Pm2, not taking into account the heat exchanges with the outside, is expressed as:
$$w_{\text{Pm2}} = \frac{{\dot{W}_{\text{Pm2}} }}{{\dot{m}}} = \frac{{P_{6} - P_{5} }}{{\rho_{\text{W}} \cdot \eta_{\text{Pm2}} }} = h_{6} - h_{5}$$ -
Entropy in point 6 is expressed as:
$$h_{6} = h_{5} + \frac{{P_{6} - P_{5} }}{{\rho_{\text{W}} \cdot \eta_{\text{Pm2}} }}$$ -
The work of reversible pump Pm2 in steady state is given as:
$$w_{\text{r,Pm2}} = (h_{5} - h_{6} ) - T_{0} \cdot (S_{5} - S_{6} )$$
Pump 3:
-
The first law of thermodynamics steady volume for the water control of maple contained between sections 7 and 8 of the pump Pm3, not taking into account the heat exchanges with the outside, is expressed as:
$$w_{\text{Pm3}} = \frac{{\dot{W}_{\text{Pm3}} }}{{\dot{m}}} = \frac{{P_{8} - P_{7} }}{{\rho_{\text{W}} \cdot \eta_{\text{Pm3}} }} = h_{8} - h_{7}$$ -
Entropy in point 8 is expressed as:
$$h_{8} = h_{7} + \frac{{P_{8} - P_{7} }}{{\rho_{\text{W}} \cdot \eta_{\text{Pm3}} }}$$ -
The work of reversible pump Pm3 in steady state is given as:
$$w_{\text{r,Pm3}} = (h_{7} - h_{8} ) - T_{0} \cdot (S_{7} - S_{8} )$$
Hydraulic turbine:
-
The first law of thermodynamics in steady state for the brine control volume contained between sections 10 and 11 of the hydraulic turbine is expressed as:
$$w_{\text{TH}} = \frac{{\dot{W}_{\text{TH}} }}{{\dot{m}_{10} }} = h_{10} - h_{11} = \frac{{P_{10} - P_{11} }}{{\rho_{{10_{\text{SW}} }} \cdot \eta_{\text{TH}} }}$$ -
Entropy in point 11 is expressed as:
$$h_{11} = h_{10} + \frac{{P_{10} - P_{11} }}{{\rho_{{10_{\text{SW}} }} \cdot \eta_{\text{TH}} }}$$ -
The reversible work of hydraulic turbine in steady state is given as:
$$w_{\text{r,TH}} = w_{\text{TH}} - T_{0} \cdot (S_{10} - S_{11} )$$
Reverse osmosis unit:
-
The first law of thermodynamics in steady state for the volume control of liquid water contained between sections 8, 9 and 10 of the unit reverse osmosis is expressed as
$$h_{8} = r_{1} \cdot h_{9} + r_{2} \cdot h_{10}$$ -
The reversible work of the reverse osmosis unit in steady state is given as:
$$w_{\text{r,RO}} = - T_{0} \cdot (S_{8} - r_{1} \cdot S_{9} - r_{2} \cdot S_{10} )$$ -
The global efficiency of the reverse osmosis system is:
$$\begin{aligned} \eta &= - \frac{{W_{\text{net}} }}{{q_{\text{in}} }} \cdot 100 \hfill \\ W_{\text{net}} &= W_{\text{TV,real}} + W_{\text{Pm1,real}} + \frac{1}{{r_{3} }} \cdot \left( {r_{2} \cdot W_{\text{TH,real}} + W_{\text{Pm2,real}} + W_{\text{Pm3,real}} } \right) \hfill \\ \end{aligned}$$ -
The variation in efficiency versus flow mass rate (Fig. 8) has the same shape, by taking pressure for all, and the heat energy constant in each one, with a little growth in the beginning, and then it becomes constant, because of the limitation of the efficiency of system. The green, red and blue curves have maximum efficiencies of 44.76%, 30.18% and 8.308%, respectively.
Figure 9 describes the efficiency versus pressure by fixing the thermal heat of evaporator \(q_{\text{in}}\) in each curve and mass flow rate for all. They have a minimum and maximum extremity of variation. The green curve rises from 31.29 to 96.49%, red one from 20.39 to 92.26% and the blue one from 0.18 to 85.91%.
The efficiency \(\eta\) of system increases exponentially with thermal heat \(q_{\text{in}}\) of evaporator, see Fig. 10. The green curve increases from 1.87 to 89.58%, red one from 4.07 to 74.36% and the blue from 3.02 to 51.65%, as it is observed that the forms of all curves have the same shape, because of linearity of equation system.
In the latter case (system # 2), Fig. 11, the two subsystems are connected mechanically and thermally. Recovering the power unit is again a hydraulic turbine.
The heat exchanger (evaporator) effectiveness ε is a function of flow mass rate \(\dot{M}\) (kg/s) with four thermal heat constants \(q_{\text{in}}\) (kJ/kg) from 950 to 1250 kJ/kg. The four curves have the same exponential shape and distance between the them, because of linearity of the problem. The efficiency of red curve increases from 0 to 22%, the cyan from 11 to 31%, the brown from 21 to 40% and the green from 31 to 48% as shown in Fig. 12. The efficiency in Fig. 13 increases from 20 to 90% for the four curves, with different mass flow rates.
MED system model
We have worked in this paper just on the vacuum system, because it plays a key role in the regulation of the pressure in the evaporator; this improvement will be useful for the MED system (Fig. 14).
Using the results obtained from this case study, one can see that for a jet vacuum pump (Fig. 15), with liquid for creating a vacuum to a volume of 1.571 m3, a water pump is required along with a flow rate of 24 m3/h and a head of 52.23 m.
Change in the parameters has shown a lower vacuum pump (and therefore less than volume flow) will produce smaller heads and consequently you will certainly need lower vacuum pump. It means the size of required vacuum will play an essential role in selecting a vacuum pump. The selection of a vacuum pump for a certain vacuum size may be made from the datasheets provided from the manufacturer of the vacuum pump. One more observation which can be made is that the requisite head shall be smaller for the pump if the losses from domestic water are minimized.
The interface developed will give the ability of the control of all parameters required in the seizing of the vacuum system as shown in Fig. 15. The results which have been obtained from the EES program (Oulhazzan et al. 2016) can be used to choose correctly for water pump a specific liquid jet vacuum pump. It can be realized because the head (the height of the fluid to be pumped) and the volumetric flow to obtain the required vacuum have become known. Additional information for the jet vacuum pump liquid and the necessary time to achieve the required vacuum can be found in datasheet from the manufacturer of the liquid jet system of the vacuum pump. This information could then be combined together to develop a complete vacuum system for the evaporator of a desalination plant (Klein et al. 1992; Aroussy et al. 2016c, d).
Figure 16 presents the total height (H) that has to be pumped by the water system, in function of pressure entering the heater. The variations in curves are linear, just with a little curvature in the beginning; all calculus is made for different mechanical losses caused by valves and elbows in the auxiliary system. Also the volume of vacuum vessel increases significantly with the diameter for such values of lengths in Fig. 17.
By changing the input parameters, different comments may be made. For example, when the temperature of the steam is enhanced, it causes a greater overall coefficient of the heat transfer. It means that one smaller evaporator shall be required because a lower heat transfer area is necessary. A further observation which can be done is the mass flow of the saltwater is reduced; the overall coefficient of heat transfer also increases. This means that less of the product (freshwater) will be produced in a certain time (Fig. 18).
Conclusion
This study develops a combination of MED and RO for cogeneration of power plant, by heat recovery system during acid sulfuric production used in desalination process. The parametric study of vacuum system shows the effect of each one on the other (efficiency, flow mass rate thermal heat and pressure).
From the analysis carried out, we conclude that heat the coupling has a large effect on energy and exergetic performance of electrical and water cogeneration. It increases the operating temperature for the reverse osmosis unit increasing the amount of drinking water produced, although meanwhile, it influences the quality of the permeate. Therefore, we should not raise this working temperature to a maximum value which corresponds to the permeate salinity of 1545 ppm. On these conditions, we obtain the optimum operation of the system which has the maximum energy efficiency and exergy.
References
Amry Y, El Gaidi M, Nachtane M (2016). Design and optimization of a smart meter to meet the growing needs of energy in morocco. In: 2016 international renewable and sustainable energy conference (IRSEC), Nov 2016, IEEE. pp 1115–1118
Aroussy Y, Nachtane M, Oulhazzan M, Tarfaoui M, Saifaoui D (2016a) Exergy analysis of a MSF-MED desalination plant for optimal design. Eur J Sci Res 142:29–39
Aroussy Y, Nachtane M, Saifaoui D, Tarfaoui M, Rouway M (2016b) Thermodynamic performance evaluation of a reverse osmosis and nanofiltration desalination. Int J Appl Eng Res 11(17):9149–9153
Aroussy Y, Nachtane M, Saifaoui D, Tarfaoui M (2016c) Numerical investigation of a reverse osmosis desalination system with cogeneration and renewable energy integration. Int J Sci Eng Res 7:6
Aroussy Y, Nachtane M, Saifaoui D, Tarfaoui M, Farah Y, Abid M (2016d) Using renewable energy for seawater desalination and electricity production in the site OCP morocco. J Sci Arts 16(4):395
Bouzayani N, Galanis N, Orfi J (2007) Comparative study of power and water cogeneration systems. Desalination 205:243–253. https://doi.org/10.1016/j.desal.2006.03.553
Cangel YA, Boles MA (2002) Thermodynamics: an engineering approach 4th edition in SI units. McGraw-Hill, Singapore
Cardona E, Piacentino A (2004) Optimal design of cogeneration plants for seawater desalination. Desalination 166:411–426. https://doi.org/10.1016/j.desal.2004.06.096
de Gunzbourg J, Larger D (1999) Cogeneration applied to very high efficiency thermal seawater desalination plants. Desalination 125:203–208. https://doi.org/10.1016/S0011-9164(99)00139-3
El-Nashar AM (2001) Cogeneration for power and desalination—state of the art review. Desalination 134:7–28. https://doi.org/10.1016/S0011-9164(01)00111-4
Khoshgoftar Manesh MH, Ghalami H, Amidpour M, Hamedi MH (2013) Optimal coupling of site utility steam network with MED-RO desalination through total site analysis and exergoeconomic optimization. Desalination 316:42–52. https://doi.org/10.1016/j.desal.2013.01.022
Klein SA, Alvarado FL (1992) EES: engineering equation solver for the Microsoft Windows operating system. F-Chart Software
Lovato A, Legorreta C, Andersson E (2001) Heat recovery from sulphuric acid plants for seawater desalination. Desalination 136:159–168. https://doi.org/10.1016/S0011-9164(01)00178-3
Mourad N, Mostapha T, Dennoun S (2018) Promotion of renewable marines energies in Morocco: perspectives and strategies. World Acad Sci Eng Technol Int J Energy Power Eng 5(1):12
Oulhazzan M, Nachtane M, Saifaoui D, Winninger D, Aroussy Y (2016) Design and thermodynamics performance evaluation of a new stirling engine prototype. Int J Sci Eng Res 7:8
Saifaoui D, Rouway M, Nachtane M, Tarfaoui M (2016) Analytical and numerical investigation of a sea water desalination plant with integration of renewable marine energy (Jorf Lasfar OCP Morocco). In: Progress in renewable energies offshore: proceedings of the 2nd international conference on renewable energies, 2016 (RENEW2016). Taylor & Francis Books Ltd, pp 495–499
Author information
Authors and Affiliations
Contributions
MN, MT, DS, MR and YA contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this paper declare that they have no conflict of interest and compliance with ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saifaoui, D., Nachtane, M., Tarfaoui, M. et al. Heat recovery from sulfuric acid plants for seawater desalination using RO and MED systems. Appl Water Sci 10, 99 (2020). https://doi.org/10.1007/s13201-020-01182-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01182-7